Addiction Craving and Cue Reactivity Initiative Network (ACRIN)

Background
Exposing people with substance use disorders (SUDs) to addiction-related cues can engage neural circuits and processes that are fundamental to maintenance of SUDs. This multi-level physiologically and psychologically response to cues is called cue-reactivity. Investigating cue-reactivity and subsequent urges to consume drugs, which can be called cue-induced craving, has contributed to our understanding of the pathophysiology of SUDs and potential biomarkers for treatment development. There are many labs across the world that use cue reactivity paradigms in human and non-human subjects, but there has been no collaborative network to help integrate this work. The Addiction Craving and Cue Reactivity Initiative Network (ACRIN) is a Virtual Collaborative Network in Science (ViCoNS) aiming at advancing the field of addiction cue-reactivity and craving.
As a ViCoNS, the ultimate goals of ACRIN are to improve the methodological rigor and reporting standards for Addiction Craving and Cue Reactivity research studies as we strive to harness these methods to inform our understanding of the etiology of substance use and develop improved interventions. To help achieve this goal, we strive to support a culture of academic collegiality, transparency, data sharing/open science, diversity and inclusiveness. ACRIN has been active in holding workshops, publishing white papers, generating consensus statements and guidelines, and developing consortiums for mega-analyses, to promote the reproducibility, methodological rigor and advancement of the field.
Main Questions
- How assessment of craving and cue reactivity can be optimized and/or harmonized?
- How craving and cue reactivity can be recognized as surrogate measures/biomarkers of recovery?
- How evidence from craving and cue reactivity can be mapped and translated across species?
Governance
ACRIN as a virtual collaborative network in science is a platform for collaborations between members based on collegiality and inclusiveness. ACRIN does not have any form of property or rights beyond its name and logo. Both Subscription and membership to the network is free. Membership will need submission of CV and letter of interest and a basic review process by the steering committee. Members can propose new projects within ACRIN. Decisions are being made with consensus among the steering committee. Members can use ACRIN platform, name and logo after receiving written approval from the director.
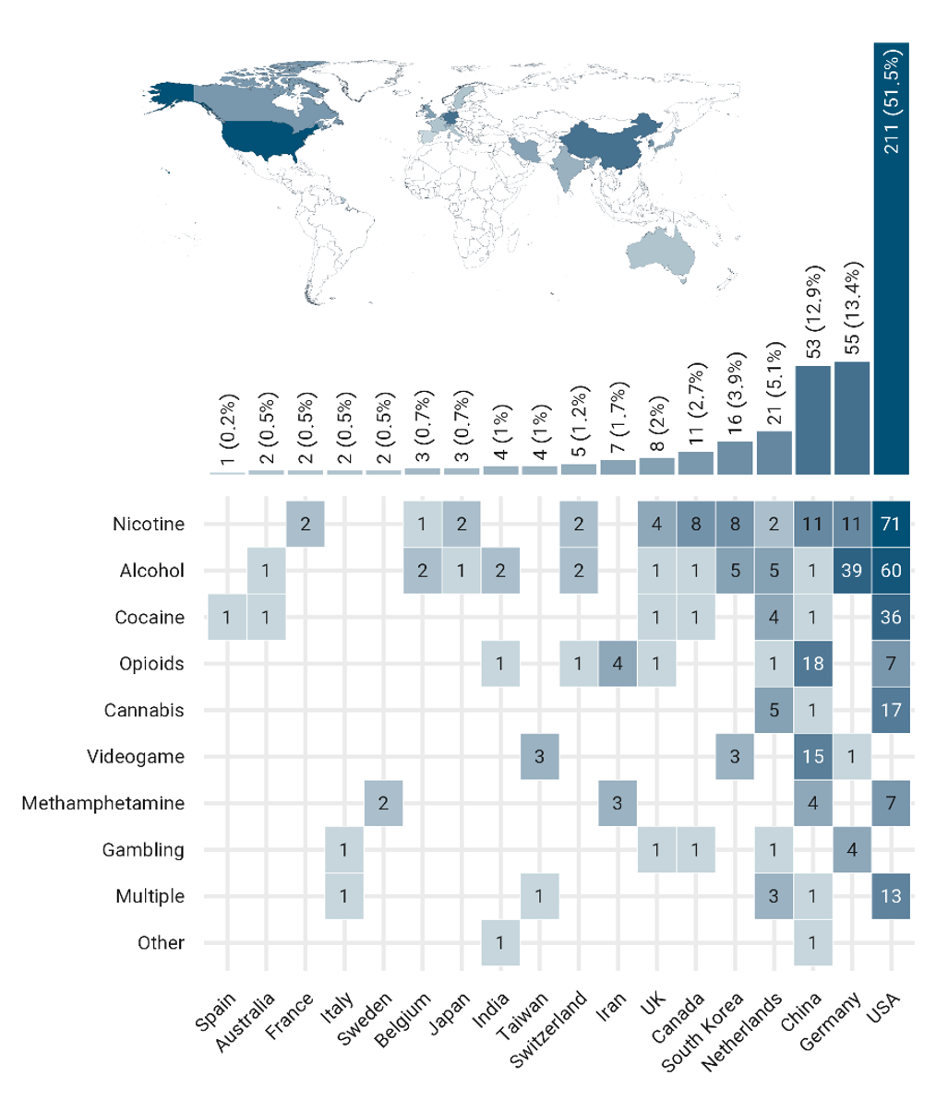
fMRI drug cue-reactivity (FDCR) is one of the areas of collaborative activities in ACRIN. There is a global contribution to FDCR studies
Number of FDCR studies in each country, broken down by the type of addictive substance/behavior. “Multiple” stands for studies including more than one type of addictive substance/behavior. The "other" category includes inhalants and betel-quid. Note that only papers whose full-text was in English were included, potentially leading to a relative over-representation of majority English-speaking countries.
Areas of Collaboration (Workstreams)
Drug Cue Databases
In recent years, there has been an increasing recognition of the role of drug cue-reactivity in addiction, wherein exposure to drug-related cues can trigger intense craving and subsequent drug-seeking behavior. Pictorial drug cue databases have emerged as valuable tools for investigating the neural and cognitive processes underlying cue-reactivity. This project aims to compare these databases and define standards for drug cue-reactivity databases, enhancing the quality and rigor of research in this field. By developing guidelines and consensus statements on how to develop validated cue databases, we hope to encourage development of new databases, increase transparency, improve quality of data acquisition, facilitate collaboration among researchers, improve data sharing, and promote advancements in our understanding of drug cue reactivity and its implications in addiction research.
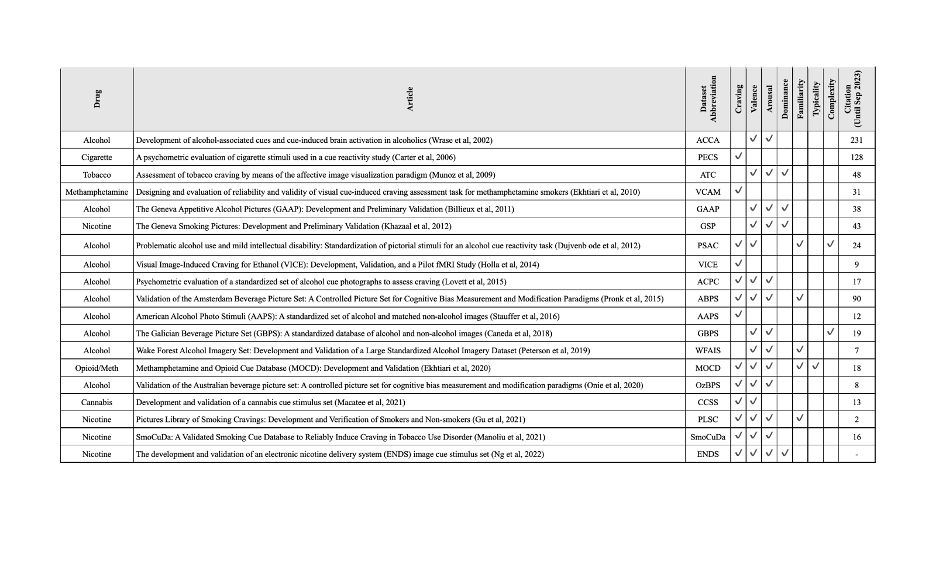
An overview on some of the available drug cue databases and their validation methods.
This is an effort to provide access for researchers and also increase the quality of cue databases that are going to be developed in the future.
Craving Self Reports
Drug craving is a dynamic emotional-appetitive state for drug use subjectively experienced as urge/craving and can be triggered in response to drug related cues. Craving is now one of the criteria for diagnosis of substance use disorders in DSM-5 and contributes to relapse to drug use in people who are in abstinence/recovery. Craving is frequently measured by single-item questionnaires, e.g., “How much craving do you experience now?”; however, several multi-item scales have also been developed to improve measurement validity. The heterogeneity of the available drug craving assessment tools have led to challenges in data aggregation and replicability assessments. There is still no consensus on a decision tree on how to select a measurement tool to assess craving as an outcome measure in a trial. We are conducting two parallel systematic reviews of craving self-reports aimed to catalog and summarize craving assessment tools registered in ClinicalTrials and PubMed, focusing on the diversity and frequency of self-report questionnaires along with their time frame and structure. We are also conducting a content analysis project on all published craving self-reports to map the constructs targeted across the available tools.
i. Craving as an Outcome Measure in Drug Addiction Trials: A Systematic Review of the ClinicalTrials.gov
This projects protocol has been submitted on https://osf.io/vmcf7/
ii. Craving as an outcome measure in trials with substance use disorder: A systematic review within PubMed (below).
This projects protocol has been submitted on https://osf.io/jcpt9/
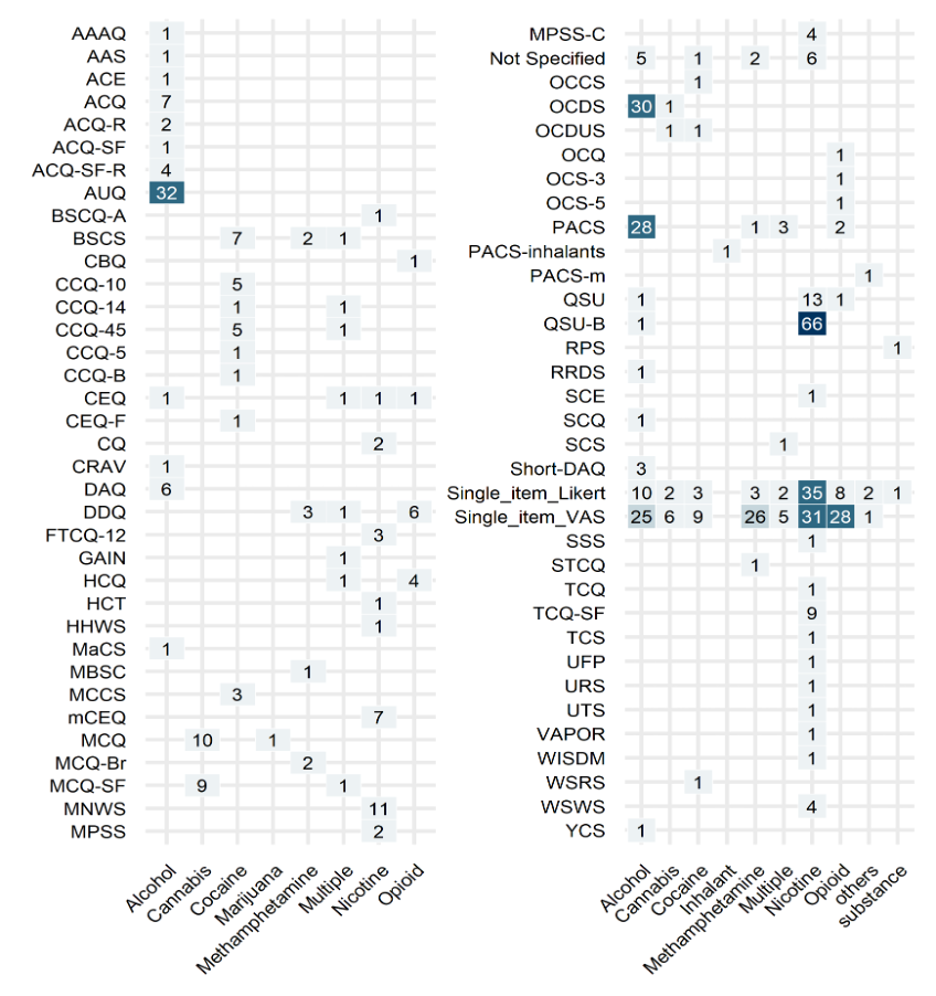
Various self-reports for craving used in clinical trials between 2016-2022.
Craving has frequently been an important outcome measure in trials, but there is no agreement on the best way to assess craving as an outcome measure in trials.
fMRI Drug Cue-Reactivity (FDCR) Tasks
FDCR studies have gained significant popularity as a means of assessing brain function in individuals with SUDs. Numerous tasks have been developed to present cues to participants in addiction research studies. However, the full range of task variations has not been thoroughly described. While acknowledging the importance of task design variations in FDCR studies, we are currently investigating the possibility of collectively proposing a narrower parameter space for FDCR tasks. This aims to enhance replicability and establish FDCR tasks as a validated outcome measure in clinical trials, particularly those focused on developing potential treatments for SUDs.
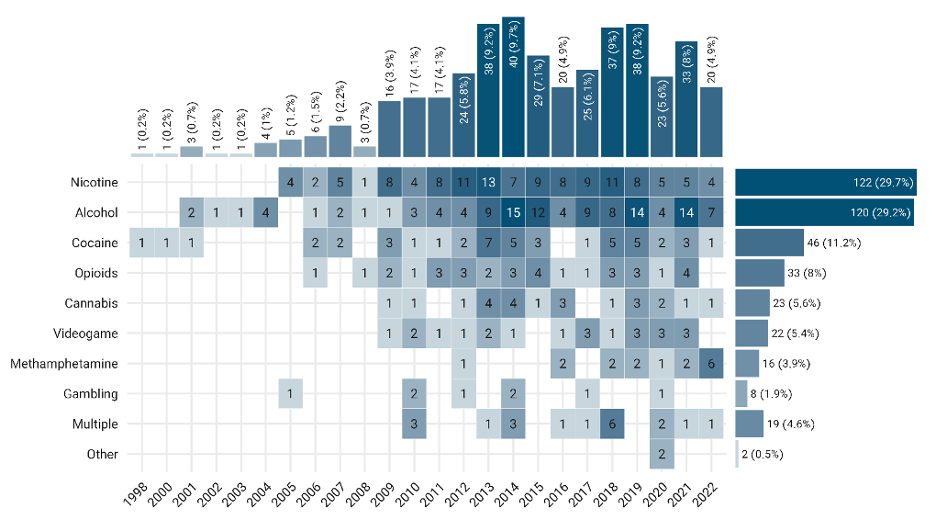
fMRI drug cue-reactivity studies from 1998 to 2022
Number of FDCR studies each year from 1998 till the end of 2022, broken down by the type of addictive substance/behavior. “Multiple” stands for those studies including more than one type of addictive substance/behavior. The "other" category includes inhalants and betel-quid.
FMRI Drug Cue Reactivity (FDCR) Consortium: ENIGMA ACRI
Cue-reactivity is one of the most frequently used paradigms in functional magnetic resonance imaging (fMRI) studies of substance use disorders (SUDs). Although there have been promising results in recent years among FDCR studies, there still are not any consortiums to test the replicability of results across labs and variations in tasks. Within the Enhancing NeuroImaging Genetics through Meta‐Analysis (ENIGMA) consortium, ENIGMA Addiction Cue-Reactivity Initiative (ENIGMA-ACRI) has started to fill this gap by starting a consortium to gathering FDCR databases to be able to do mega analyses.

Sample webinar organized by the ACRIN team to explore potentials for drug cue reactivity as a biomarker
With more than 100 attendees, this webinar discussed how to develop neuroimaging-based biomarkers for clinical trials and what is the path through FDA approval. Also, the ENIGMA ACRI checklist was presented, and speakers talked about its different aspects. The webinar concluded with ways to advance and share the FDCR studies within the ENIGMA framework for mega-analyses. Online webinars provide opportunities for worldwide collaborations among network members.
Link to October 29, 2020 ACRIN webinar: https://www.youtube.com/watch?v=U7v-FN6IY60&t=6153s
Drug Cue Reactivity Across Species
Cue-reactivity can be used across multiple species, but there is still a lack of consensus on how to optimize cross species studies. This work group is interested in bringing together PIs who believe cue reactivity studies in rodents and non-human primates can inform human studies and vice versa. This can open doors for cross-species biomarkers and treatment development for SUDs.
FDCR as a Treatment Response Biomarker
A “biomarker” as a defined characteristic measured as an indicator of normal or pathogenic biological processes, or biological responses to an exposure or intervention, including therapeutic interventions. The development of clinically relevant biomarkers is a major goal of translational addiction sciences and translational psychiatry more broadly. The literature on fMRI drug cue-reactivity (FDCR) has matured significantly in recent years, suggesting that brain activations in cue-reactivity across addictive disorders and in specific SUDs are complex phenomena. Meta-analyses of the literature have demonstrated the involvement of disparate brain regions, circuits, and networks in cue-reactivity and suggest that FDCR tasks effectively engage neural circuitry underpinning different aspects of addiction-relevant cue processing. Response biomarkers may indicate the presence of a treatment effect on neuroimaging biomarkers of recognized importance in SUDs and provide some estimate of the intensities and locations of such effects. This can also be used to screen candidate therapeutics and prioritize those with plausible effectiveness. After more than three decades and more than 400 FDCR studies, there has not been an FDA-approved biomarker that can show the effect of an intervention using FDCR. This working group is aiming at collecting enough evidence and conducting future studies to be able to fill this gap.

A sample symposium organized by the ACRIN team members
Facilitating cross talks among key stakeholders and scientists is one of the main goals in the network. The panel Brains Before Brawn: Using Brain-Based, Outcome-Relevant, Endophenotypes in Phase 2 Medication Development for Psychiatric Disorders-to Improve Our Success in Larger Phase 3 Clinical Trials was held at the prestigious 60th Annual Meeting of the American College of Neuropsychopharmacology (ACNP). The panel highlighted efforts with brain endophenotypes for anhedonia and suicidality in depression and for reward, inhibitory, and stress circuitry dysfunction in SUDs. The comments on the utility of endophenotypes from the perspective of the pharmaceutical industry were discussed. Finally, there was a discussion on the several parallels in circuitry dysfunction across discussed disorders.
Abstract: https://scholar.google.com/scholar?cluster=129403002452588743&hl=en&oi=scholarr;
Recording: https://www.dropbox.com/s/24xr981k1g80iim/ACNP_2021-Brains%20Before%20Brawn.avi?dl=0
FDCR as a Neuromodulation Target Biomarker
Mechanistic studies and clinical trials have provided increasing evidence for the effectiveness of non-invasive neuromodulation with transcranial electrical (tES) or magnetic stimulation (TMS) in the treatment of people with SUDs, including SUDs related to alcohol, tobacco, cocaine, cannabis, methamphetamine, and opioids. The enthusiasm for non-invasive neuromodulation approaches to SUD treatment is buoyed by a growing body of work demonstrating an important relationship between non-invasive stimulation of the frontal-striatal circuits and drug-related behaviors. At the end of 2018, only 84 published tES/TMS trials had been conducted in the field of SUDs. By the end of 2022, 205 tES/TMS trials had been published in the treatment of SUDs. Following a decade of rapid growth and expansion of the non-invasive neuromodulation tools into the SUD research field, the United States Food and Drug Association (FDA) approved TMS for smoking cessation (FDA: K200957) in 2020. The supporting multicenter, double-blind, randomized controlled trial included 135 participants with tobacco use disorders and showed that the active rTMS group had significantly higher smoking abstinence rates at weeks 2, 4, and 12 compared to the sham group. Since then, there has been an accelerating pace of new clinical trials using novel tools and protocols of non-invasive neuromodulation for SUDs, and the list of devices and indications that have received CE marking in Europe is growing. Cue reactivity and craving have become an important part of both assessment and intervention in tES/TMS trials in SUDs. The FDA clearance for TMS for smoking cessation includes the “cue exposure” as a part of the treatment protocol. In this workgroup, we hope to optimize using FDCR as a target biomarker.
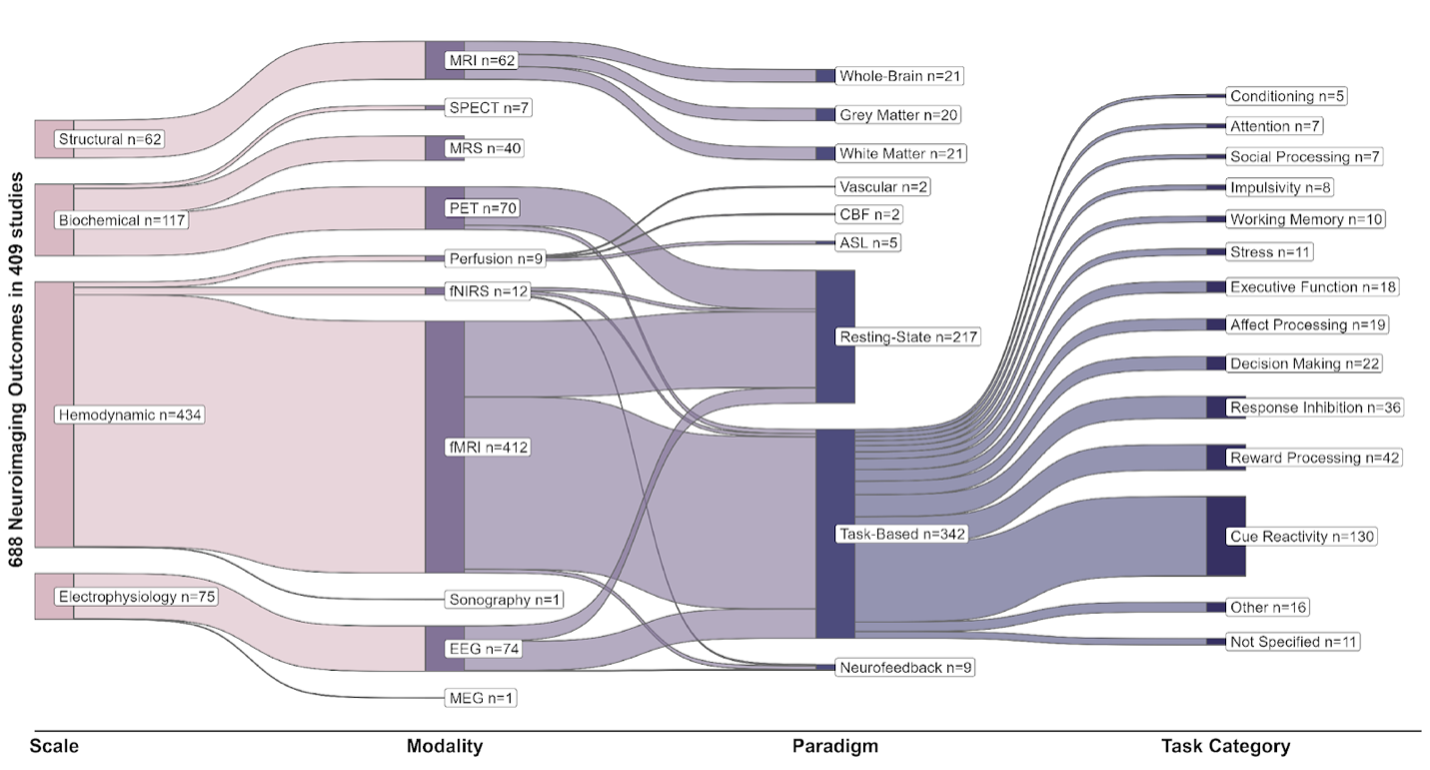
Multi-level characteristics of 688 neuroimaging outcome measures in 409 registered trials
These levels include the scales at which neuroimaging modalities have probed the nervous system (structural, biochemical, hemodynamic or electrophysiology), the neuroimaging modality, different paradigms in each modality, and the types of tasks used in task-based functional neuroimaging paradigms. All “structural” paradigms in our database were variants of MRI; “biochemical” paradigms include SPECT, MRS and PET; “hemodynamic” paradigms include fMRI, fNIRS, less common perfusion imaging modalities and ultrasound; and EEG and MEG constitute “electrophysiological” imaging paradigms. These modalities have been used for static structural scans of brain gray or white matter and vasculature, resting-state functional scans, or task-related functional scans with various tasks. Note that many studies have utilized more than one imaging paradigm and the total number of paradigms is 688, more than the number of trials (n=409).
Cue-Based Cognitive/Behavioral Interventions
Exposure to drug-related cues could elicit conditioned responses (e.g., drug craving) which can lead to relapse even in people who had formerly used substances and are in long-term abstinence. Drug cues may activate cognitive and neural correspondence to addiction and promote responsiveness to interventions. Furthermore, to increase ecological validity, real-world drug cues could be integrated within various models of addiction interventions. Applying different models of cue-based interventions (e.g., cue-exposure therapy, cognitive-bias modification) could potentially reduce cue sensitivity and cue reactivity and subsequently lead to lower relapse rates. This set of interventions engage various cognitive processes ranging from basic (e.g., attention, working memory) to more complex (e.g., flexibility, future thinking) ones. The Cue-Based Interventions subnetwork of ACRIN aims to collect and review the existing evidence, build consensus and guidelines on clinical and mechanical framework for cue-based interventions, and synergize activities across members’ labs.
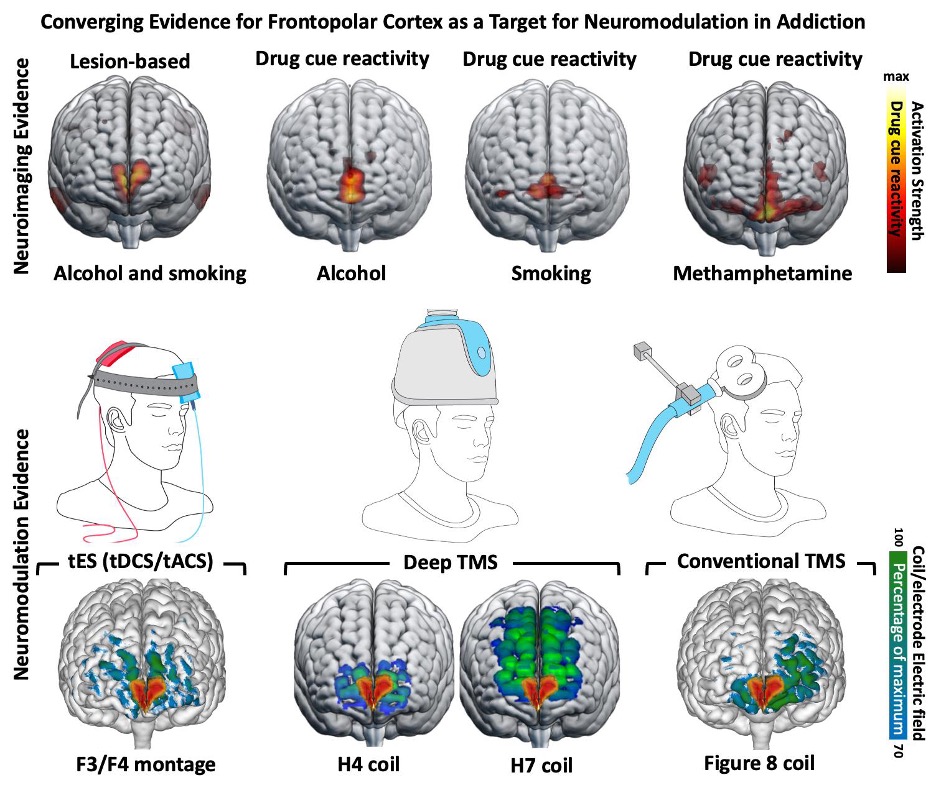
Multiple levels of evidence supporting cue reactivity in frontopolar cortex as a biomarker across various SUDs
There is an ongoing effort to optimize fMRI drug cue reactivity as a target biomarker in various non-invasive brain stimulation interventions. https://ajp.psychiatryonline.org/doi/10.1176/appi.ajp.20221022
ACRIN Publications
ACRIN FDCR Checklist
Cue-reactivity is one of the most frequently used paradigms in functional magnetic resonance imaging (fMRI) studies of substance use disorders (SUDs). Although there have been promising results elucidating the neurocognitive mechanisms of SUDs and SUD treatments, the interpretability and reproducibility of these studies are limited by incomplete reporting of many important aspects. Therefore, a consensus paper and Delphi study was conducted among 45 prominent scientists in the field of fMRI Drug Cue Reactivity (FDCR) from around the world led by several members of the ACRIN team. This checklist presented structured recommendations for more comprehensive methods reporting and reviewed the FDCR literature to assess the reporting of items that are deemed important. Published on nature protocols (https://www.nature.com/articles/s41596-021-00649-4) this checklist can help improve experimental design, reporting and the widespread understanding of the FDCR protocols. This checklist can also provide a sample for developing consensus statements for protocols in other areas of task-based fMRI.
Parameter Space and Potential for Biomarker Development in 25 Years of fMRI Drug Cue Reactivity White Paper
In the last 25 years, hundreds of fMRI drug cue-reactivity (FDCR) studies in people with substance use disorders (SUDs) have formed an extensive body of evidence characterizing the neural substrates of drug-related processing bias in addictions. However, no FDCR-derived biomarker has obtained FDA drug development approval or clinical adoption. Traversing this translational gap requires a systematic assessment of the available evidence, mapping of the methodological heterogeneity of FDCR, and an evaluation of possible clinical uses of FDCR-derived biomarkers to focus research and reliability/validation efforts. This systematic review of 415 FDCR studies published from 1998 up to 2022 summarizes the state of the field, provides a systematic assessment of their potential for biomarker development for the first time, and outlines the biomarker validation process to guide future validation efforts. Based on this systematic review and a biomarker development framework, we outlined a pathway for the development and regulatory qualification of FDCR-based biomarkers of addiction and recovery. Further validation and cost-benefit analyses could support the use of FDCR-derived biomarkers clinically, potentially accelerating treatment development and improving diagnostic and prognostic judgments.
This is a living systematic review which is being annually updated, for 2023 updates, click here and then go to the HTML webpage.
ACRIN Steering Committee
- Anna Rose Childress, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
- Hamed Ekhtiari, Department of Psychiatry & Behavioral Sciences, University of Minnesota, Minneapolis, MN, USA
- Rita Goldstein, Departments of Psychiatry and Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY, USA
- Andreas Heinz, Department of Psychiatry and Neurosciences, Charite Campus Mitte, Charité–Universitätsmedizin Berlin, Berlin, Germany
- Amy Janes, National Institute of Mental Health, Baltimore, MD, USA
- Jane Joseph, Department of Neuroscience, Medical University of South Carolina, Charleston, SC, USA
- Hedy Kober, Department of Psychiatry, Yale School of Medicine, New Haven, CT, USA
- Joseph McClernon, Department of Psychiatry and Behavioral Sciences, Duke University, Durham, NC, USA
- Martin Paulus, Laureate Institute for Brain Research, Tulsa, OK, USA
- Lara Ray, Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, CA, USA
- Rajita Sinha, Department of Psychiatry, Yale School of Medicine, New Haven, CT, USA
- Elliot Stein, Intramural Research Program, National Institute on Drug Abuse, Baltimore, MD, USA
- Reagan Wetherill, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
- Anna Zilverstand, Department of Psychiatry & Behavioral Sciences, University of Minnesota, Minneapolis, MN, USA
ACRIN Members
Members and Affiliations
| Name | Affiliation | Country |
| Raymond Anton | Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, USA |
United States |
| Patrick Bach | Department of Addictive Behaviour and Addiction Medicine, Central Institute of Mental Health (CIMH), Heidelberg University, Mannheim, Germany |
Germany |
| Alex Baldacchino | Division of Population Studies and Behavioural Sciences, St Andrews University Medical School, Scotland, UK |
United Kingdom |
| Anne Beck | Department of Psychiatry and Psychotherapy, Charité, Universitätsmedizin Berlin, Campus Charité Mitte, Berlin, Germany |
Germany |
| James Bjork | Department of Psychiatry, Virginia Commonwealth University |
United States |
| Judson Brewer | Department of Behavioral and Social Sciences, Brown University School of Public Health, Providence, RI, USA |
United States |
| Anna Rose Childress | Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA |
United States |
| Eric Claus | Department of Biobehavioral Health, The Pennsylvania State University, University Park, PA, USA |
United States |
| Kelly Courtney | Department of Psychiatry, University of California, San Diego, La Jolla, CA, USA |
United States |
| Mohsen Ebrahimi | Iranian National Center for Addiction Studies (INCAS), Tehran University of Medical Sciences, Tehran, Iran |
Iran |
| Hamed Ekhtiari | Laureate Institute for Brain Research Department of Psychiatry & Behavioral Sciences, University of Minnesota, Minneapolis, MN, USA |
United States |
| Ali Fathi | Iranian National Center for Addiction Studies (INCAS), Tehran University of Medical Sciences, Tehran, Iran |
|
| Francesca Filbey | Center for BrainHealth, School of Behavioral and Brain Sciences, University of Texas at Dallas, Dallas, TX, USA |
United States |
| Dara G. Ghahremani | Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, CA, USA |
United States |
| Peyman Ghobadi Azbari | Iranian National Center for Addiction Studies (INCAS), Tehran University of Medical Sciences, Tehran, Iran |
Iran |
| Rita Goldstein | Departments of Psychiatry & Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY, USA |
United States |
| Anneke Goudriaan | Department of Psychiatry, Amsterdam University Medical Center, Amsterdam, The Netherlands |
Netherlands |
| Erica Grodin | Department of Psychology, University of California Los Angeles, Los Angeles, CA, USA |
United States |
| Paul Hamilton | Center for Medical Image Science and Visualization, Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden |
Sweden |
| Colleen A. Hanlon | Department of Cancer Biology, Wake Forest School of Medicine, Winston-Salem, NC, USA |
United States |
| Peyman Hasani Abharian | Brain and Cognition Clinic, Institute for Cognitive Science Studies, Tehran, Iran |
Iran |
| Andreas Heinz | Department of Psychiatry and Neurosciences, Charité Campus Mitte, Charité–Universitätsmedizin Berlin, Berlin, Germany |
Germany |
| Amy Janes | National Institute of Mental Health | United States |
| Jane E. Joseph | Department of Neuroscience, Medical University of South Carolina, Charleston, SC, USA |
United States |
| Marc J. Kaufman | McLean Hospital/Harvard Medical School | United States |
| Falk Kiefer | Department of Addictive Behaviour and Addiction Medicine, Central Institute of Mental Health (CIMH), Heidelberg University, Mannheim, Germany |
Germany |
| Arash Khojasteh Zonoozi | Iranian National Center for Addiction Studies (INCAS), Tehran University of Medical Sciences, Tehran, Iran |
Iran |
| Hedy Kober | Department of Psychiatry, Yale School of Medicine, New Haven, CT, USA |
United States |
| Steven Kohut | Department of Psychiatry, Harvard Medical School |
|
| Rayus Kuplicki | Laureate Institute for Brain Research, Tulsa, OK, USA |
United Sates |
| Qiang Li | Department of Radiology, Tangdu Hospital, Fourth Military Medical University, Xi’an, China |
China |
| Edythe D. London | Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, CA, USA |
United States |
| Joseph McClernon | Department of Psychiatry and Behavioral Sciences, Duke University, Durham, NC, USA |
United States |
| Hamid Noori | McGovern Institute for Brain Research, Massachusetts Institute of Technology, Cambridge, MA, USA |
USA |
| Jason Oliver | Department of Psychiatry & Behavioral Sciences, Oklahoma State University Center for Health Sciences, Tulsa, OK, USA |
United States |
| Max Owens | Department of Psychiatry, University of Vermont, Burlington, VT, USA |
United States |
| Martin Paulus | Laureate Institute for Brain Research, Tulsa, OK, USA |
United States |
| Irene Perini | Center for Social and Affective Neuroscience, Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden |
Sweden |
| Marc Potenza | Department of Psychiatry, Yale School of Medicine, New Haven, CT, USA |
United States |
| Stephane Potvin | Centre de recherche de l’Institut Universitaire en Santé Mentale de Montréal, University of Montreal, Montreal, Canada |
Canada |
| James J Prisciandaro | Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, USA |
United States |
| Parnian Rafei | Trinity College Institute of Neuroscience (TCIN), Trinity College Dublin, Dublin, Ireland |
|
| Lara Ray | Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, CA, USA |
United States |
| Arshiya Sangchooli | Cognitive Neuroscience and Computational Psychiatry Lab, Melbourne School of Psychological Sciences, The University of Melbourne, Melbourne, Australia |
Australia |
| Joseph Schacht | University of Colorado Anschutz Medical Campus, Aurora, CO, USA |
United States |
| Dongju Seo | Department of Psychiatry, Yale School of Medicine, New Haven, CT, USA |
United States |
| Rajita Sinha | Department of Psychiatry, Yale School of Medicine, New Haven, CT, USA |
United States |
| Michael N. Smolka | Department of Psychiatry, Technische Universität Dresden, Dresden, Germany |
Germany |
| Rainer Spanagel | Institute of Psychopharmacology, Central Institute of Mental Health, Mannheim, Germany |
Germany |
| Vaughn Steele | Department of Psychiatry, Yale School of Medicine, New Haven, CT, USA |
United States |
| Elliot Stein | Intramural Research Program, National Institute on Drug Abuse, Baltimore, MD, USA |
United States |
| Sabine Steins Loeber | Department of Clinical Psychology and Psychotherapy, Otto-Friedrich-University of Bamberg, Bamberg, Germany |
Germany |
| Susan Tapert | Department of Psychiatry, University of California, San Diego, La Jolla, CA, USA |
United States |
| Mark Thomas | Department of Neuroscience, University of Minnesota, Minneapolis, MN, USA |
|
| Antonio Verdejo-Garcia | Turner Institute for Brain and Mental Health, Monash University, Clayton, Australia |
Australia |
| Sabine Vollstaedt-Klein | Department of Addictive Behaviour and Addiction Medicine, Central Institute of Mental Health (CIMH), Heidelberg University, Mannheim, Germany |
Germany |
| Reagan Wetherill | Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA |
United States |
| Stephen Wilson | Department of Psychology, The Pennsylvania State University, University Park, PA, USA |
United States |
| Katie Witkiewitz | Department of Psychology, University of New Mexico, Albuquerque, NM, USA |
United States |
| Torsten Wüstenberg | Dept. of Psychiatry and Psychotherapy, Charité Campus Mitte, Charité - Universitätsmedizin Berlin, Charitéplatz Berlin, Germany |
Germany |
| Kai Yuan | School of Life Science and Technology, Xidian University, Xi’an, China |
China |
| Mehran Zare-Bidoky | Neurocognitive Lab, Iranian National Center for Addiction Studies (INCAS), Tehran University of Medical Sciences |
Iran |
| Xiaochu Zhang | Department of Psychology, School of Humanities and Social Science, University of Science and Technology of China, Anhui, China |
China |
| Anna Zilverstand | Department of Psychiatry & Behavioral Sciences, University of Minnesota, Minneapolis, MN, USA |
United States |
How to connect
Email (enigmaacri@gmail.com), Twitter (@ENIGMA_ACRI), YouTube (@enigmaacri7609)