Zimmer Lab

My educational interests are microbiology/parasitology, global infectious disease, and reintegrating the various disciplines of biology.
The focus of our molecular parasitology laboratory is trypanosomes. Species of these vector-borne protozoan parasites cause World Health Organization-designated Neglected Tropical Diseases that are endemic to poor, rural populations and for which new drug targets are urgently needed. The laboratory studies aspects of their metabolism and mitochondrial gene expression, looking not only for vulnerabilities that may serve as new drug targets, but also at processes that can inform our fundamental understanding of eukaryotic evolution.
Some Trypanosomes Cause Human Diseases
The trypanosome subspecies Trypanosoma brucei brucei, infective to cattle, is non-infectious to humans and is the most utilized trypanosome model system. This genetically malleable trypanosome is convenient for exploring basic facets of trypanosome molecular biology and biochemistry. Other T. brucei subspecies, T. brucei rhodesiense and gambiense are the causative agents of African Sleeping Sickness endemic to parts of Africa.
T. brucei is one of the trypanosome species we study. Trypanosoma cruzi, causative agent of Chagas disease and endemic to South and Central America, is another. Heart disease is a common Chagas disease outcome. In this U.S., this of particular concern to some of our immigrant communities. T. cruzi has a very different life cycle than T. brucei and for this reason there are topics we specifically want to study in T. cruzi rather than T. brucei. We study multiple strains of T. cruzi which show differences in degree of infectivity and disease progression. Other trypanosomes that we study are parasitic to insect and/or other non-mammalian hosts and do not cause human disease. Genomic and phenotypic comparisons of these organisms can serve as tools to learn what is special about the trypanosomes that are able to establish symptomatic infections in humans and other relevant species. The other trypanosomes in our laboratory include species of the genera Leptomonas, Leishmania, and Vickermania.
Trypanosomes: Masters at Reinventing Themselves
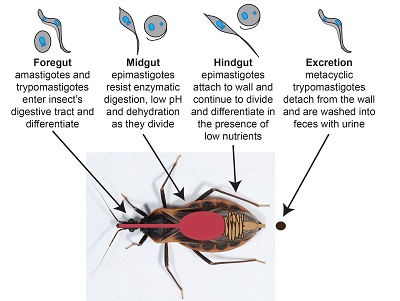 Many parasitic human pathogens are vector transmitted including T. cruzi, T. brucei, and the closely related Leishmania spp. T. cruzi is transmitted by the “kissing bug” and T. brucei by the tsetse fly. The environments of the human and insect hosts differ in temperature, pH, immune defenses, osmolarity, and nutrient availability. Therefore, trypanosomes transition through different life stages at which they are adapted to cope with the unique conditions of that stage (diagrams adjacent and below). Often the same trypanosome species in different life stages appear morphologically very different, and metabolism differs to cope with the nutrient availability of the environment. We study the trypanosome metabolic and gene expression changes that are necessary for survival at different life stages, and survival within a single host under changing conditions.
Many parasitic human pathogens are vector transmitted including T. cruzi, T. brucei, and the closely related Leishmania spp. T. cruzi is transmitted by the “kissing bug” and T. brucei by the tsetse fly. The environments of the human and insect hosts differ in temperature, pH, immune defenses, osmolarity, and nutrient availability. Therefore, trypanosomes transition through different life stages at which they are adapted to cope with the unique conditions of that stage (diagrams adjacent and below). Often the same trypanosome species in different life stages appear morphologically very different, and metabolism differs to cope with the nutrient availability of the environment. We study the trypanosome metabolic and gene expression changes that are necessary for survival at different life stages, and survival within a single host under changing conditions.
We Explore How Trypanosome Mitochondrial Gene Expression and the Composition of the Glycosome Changes in Response to Life Stage or Environmental Cues
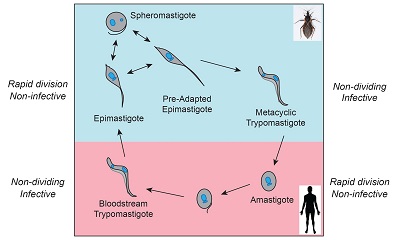 Changing metabolic pathways due to changing nutrient accessibility make it likely that changes in metabolic gene expression occur throughout trypanosome life cycles. Many genes of the trypanosome mitochondrial genome encode components of the Electron Transport Chain, and others are required to sustain this important organelle, so much of our work focuses on its gene expression. RNA stability and a unique type of RNA editing are two control points for regulating abundance of mitochondrial gene products. We are also interested in the role that compartmentalization of several normally cytosolic metabolic enzymes into an organelle called a glycosome plays in the success of these organisms as parasites.
Changing metabolic pathways due to changing nutrient accessibility make it likely that changes in metabolic gene expression occur throughout trypanosome life cycles. Many genes of the trypanosome mitochondrial genome encode components of the Electron Transport Chain, and others are required to sustain this important organelle, so much of our work focuses on its gene expression. RNA stability and a unique type of RNA editing are two control points for regulating abundance of mitochondrial gene products. We are also interested in the role that compartmentalization of several normally cytosolic metabolic enzymes into an organelle called a glycosome plays in the success of these organisms as parasites.
Example Projects
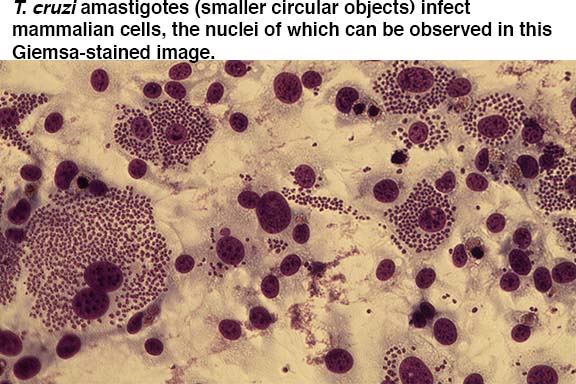 One focus of the lab is to understand how and why mitochondrial gene expression changes in T. cruzi as it progresses through its life cycle. We use cell culture, trypanosome cell biology techniques, quantitative RT-PCR, next-generation sequencing, and microscopy to test our hypotheses.
One focus of the lab is to understand how and why mitochondrial gene expression changes in T. cruzi as it progresses through its life cycle. We use cell culture, trypanosome cell biology techniques, quantitative RT-PCR, next-generation sequencing, and microscopy to test our hypotheses.
Another focus is to better understand the role that a mitochondrial mRNA cis-acting element, 3’ non-encoded tails, plays in all aspects of mitochondrial gene expression. We work in T. brucei and use and both standard molecular techniques and next-generation sequencing approaches to answering these questions.
We also work to understand the evolutionary impetus for the establishment and continued existence of compartmentalization of metabolic processes in the unique glycosome organelle of diplonema and kinetoplastids (the larger group that includes trypanosomes). We use comparative biology, cell culture, trypanosome cell biology techniques, biochemical assays and genetic manipulation in non-model trypanosomes to test our hypotheses.

|
Hina Durrani, MS Graduate Student (PhD) |

|
Austin Petersen |

|
Nooreen Sayeda |
Recent Zimmer Lab Updates
February 2022: PhD student Hina Durrani was awarded the Graduate School’s Frieda M. Kunze Fellowship for the 2022-23 academic year.
July 2021: PhD students Hina Durrani and Clara Smoniewski give great oral presentations at the 72nd Annual Midwestern Conference of Parasitologists, where Hina wins the George A. LaRue Award for the best oral presentation.

October 2020: PhD student Hina Durrani is awarded the Medical School, Duluth Campus’s 2020 Annette Boman Women’s Fellowship.
September 2020: PhD students Hina Durrani and Clara Smoniewski and undergraduates Austin Petersen and Andie Zuelke present posters at the virtual North Central Branch of the American Society for Microbiology 2020 Meeting. Clara Smoniewski places 2nd in the poster competition.
May 2020: PhD student Clara Smoniewski participates in the virtual open science hackseqRNA: COVID-19 Ultra-hackathon
February 2020: Dr. Zimmer visits collaborators at University of Ostrava and presents the talk “Probing eukaryotic rules of life with trypanosomatid parasites”.
January 2020: PhD student Clara Smoniewski was awarded the Graduate School’s Frieda M. Kunze Fellowship for the 2020-21 academic year.
October 2019: PhD student Hina Durrani presents a talk and Clara Smoniewski presents a poster at the North Central Branch of the American Society for Microbiology 2019 Meeting. Clara Smoniewski places 1st in the poster competition.
July 2019: Dr. Zimmer is promoted to Associate Professor with tenure.
July 2019: PhD student Clara Smoniewski is awarded the Department of Biomedical Sciences Johnson Fellowship
April 18, 2019: Undergraduate Sean Faacks presents his poster "Regulatory elements' effect on gene expression in Trypanosoma brucei" at the UMD Undergraduate Research & Artistic Showcase.
April 1, 2019: Congratulations to Emily Susa for a successful Masters thesis defense entitled "Gene expression within the fluctuating life cycle stages of Trypanosome parasites".
March 29, 2019: Congratulations to undergraduate Alyssa Schuett, who was awarded a UROP Fellowship for Fall 2019 for her proposed project determining the activity of the putative trypanosome KPAP2 poly(A) polymerase.
March 19, 2019: Emily Susa presents a campus-wide general-audience Grad Talk entitled "Trypanosoma cruzi parasite, master of self-reinvention"
November 19, 2018: Congratulations to undergraduate Sean Faacks, who was awarded a UROP Fellowship for Spring 2019 for his proposed project about RNA binding protein domains in T. brucei.
October 26, 2018: PhD candidate Clara Smoniewski presents her poster on trypanosome mitochondrial poly(A) polymerases and their activities at the Rustbelt RNA Meeting in Columbus, OH.
June 18, 2018: Dr. Roger Ramirez-Barrios is awarded a 2018 Para Tryp travel fellowship to present a talk at the American Association of Veterinary Parasitologists 63rd Annual Meeting in Denver, CO in July.
May 14, 2018: Master of Science candidate Charles Liggett will give his public thesis seminar entitled "Powerhouse in Flux: Changes in Mitochondrial RNA Proessing During Starvation in Trypanosoma cruzi". 3PM, Room 130, School of Medicine.
April 27, 2018: The Zimmer laboratory is well-represented in the Integrated Biosciences Art Show. Thank you Emily and Clara for contributing.
January 10, 2018: The Zimmer laboratory is grateful for a grant from the NIH-National Institute of Allergy and Infectious Diseases awarded to Dr. Sara Zimmer for investigation of post-transcriptional nucleotide addition on trypanosomatid mitochondrial mRNAs.
Name: Sara L. Zimmer, PhD
Principle Investigator, Associate Professor
Title: Principal Investigator
Email: szimmer3@umn.edu
Phone: 218-726-6741
Office Address:
Department of Biomedical Sciences
325 SMed
1035 University Drive
Duluth, MN 55812-3031
