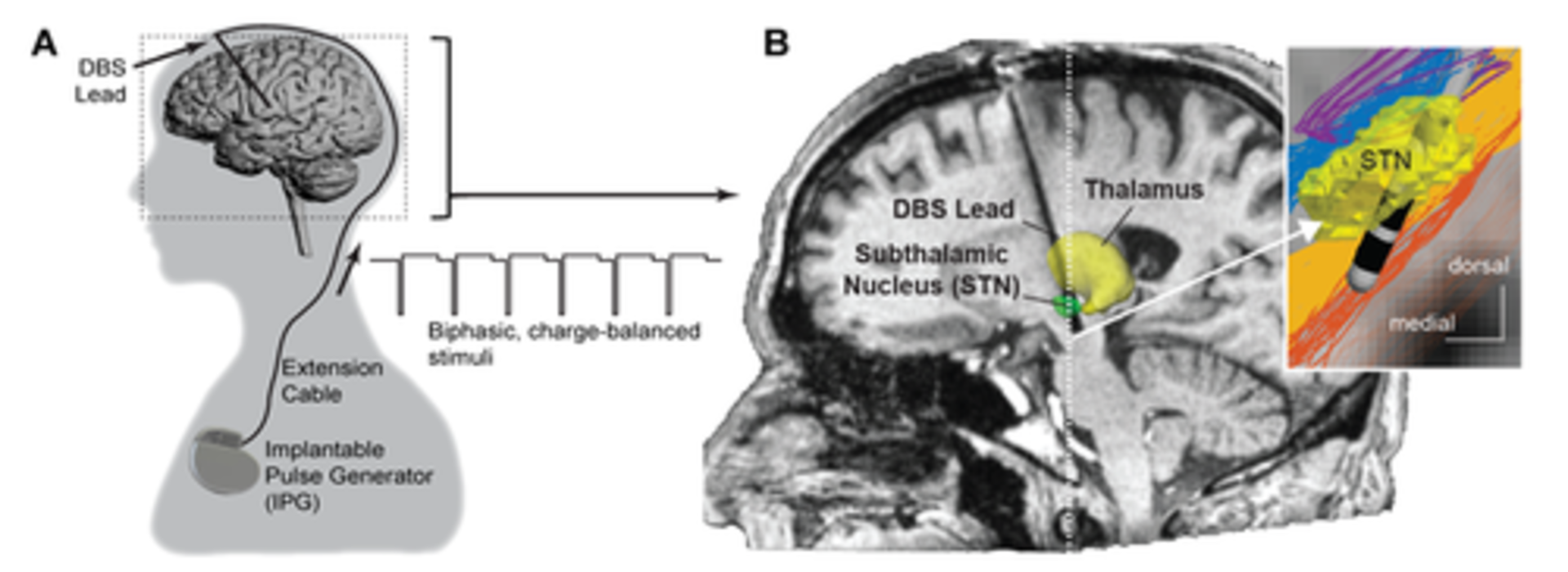
Engineering Parkinson’s Treatment
Deep brain stimulation (DBS)—implanting electrodes in the brain to regulate abnormal neural impulses—has improved the lives of countless people with Parkinson’s disease. But that doesn’t mean the procedure itself can’t be improved.
“In a lot of ways, stimulation isn’t a ‘clean’ tool,” says Jerrold Vitek, head of the Neurology Department in the Medical School and director of the University’s Udall Center of Excellence for Parkinson’s Research, which focuses on DBS therapies. “Any fiber pathways that are projecting from, adjacent to, or running through the target(s) where we put these leads are likely to get activated.”
But where should the neurosurgeon place the lead (the coated wire with electrodes near the tip) so that it truly addresses the patient’s symptoms? “We’re working to understand how DBS alters activity in the brain’s circuitry that is involved in the development of Parkinson’s disease, what changes are related to the improvement in these symptoms, and which ones may lead to side effects,” Vitek says. Simply stated, the goal is to find out which axons need to be activated and which need to be avoided.
To gain a deeper understanding of that circuitry, the Center’s neurologists, neuroscientists, and scientists in the imaging center work closely with engineers, whose tools and models are helping optimize electrode placement and performance.
A Better Map
“Parkinson’s is often thought of as a single disease,” says Matt Johnson, a professor in the U of M’s Department of Biomedical Engineering specializing in neuroscience. “But it’s actually a spectrum of symptoms.” What’s more, those symptoms vary from patient to patient. For some, tremors are more prevalent. For others, it’s slowness of movement or postural instability.
In collaboration with Vitek and professor of radiology and neurosurgery Noam Harel, Johnson and his team of engineers develop patient-specific computer models that predict how stimulation will affect brain tissue and how that can relate to treatment. In applying DBS, Johnson notes, “some symptoms are relatively easier to treat, such as tremor or slowness of movement. The ones that are harder to affect are those that subserve gait dysfunction and postural instability. And those are the ones that lead to falls and dramatic changes in quality of life.”
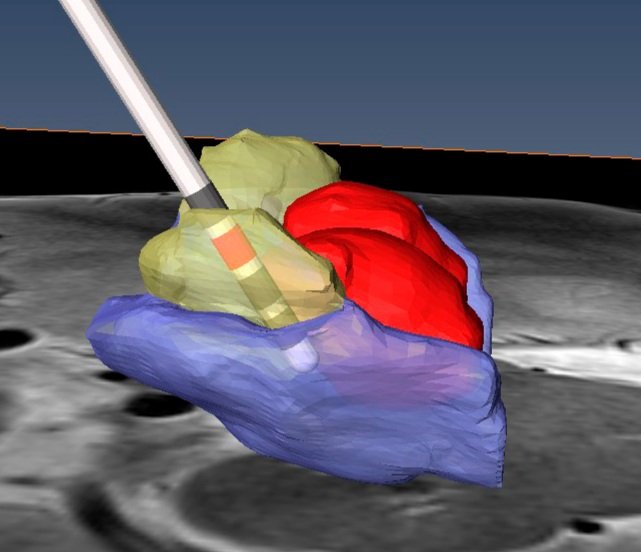
One way that Johnson and his lab help neurologists identify those pathways is by predicting how electric fields “distribute in the brain when stimulation is applied and how those electric fields influence the biophysical dynamics of fiber pathways,” he says. The computer-generated simulations also help neurologists and neurosurgeons understand – before the electrodes are implanted – how likely DBS lead implant locations and orientations will activate a particular pathway.
But to determine the best lead locations, bioengineers and neurosurgeons need detailed images of patient brains. This is where the work of neuroscientist Harel, who specializes in high-field MRI imaging, has been crucial.
Before higher-resolution imaging was readily available, neurosurgeons would use an image of a patient’s brain and merge it with what Harel describes as an “atlas”—a map of the human brain derived from the brains of two French Parkinson’s patients. The problem with such an atlas, he adds, is “that my brain and your brain aren’t exactly the same.” Now, with more precise imaging that can map the structures distinctive to each patient's brain, “we can actually visualize the target where the neurosurgeon needs to go,” Harel says. After the surgery, images can be fed into the engineers’ model so that the programming neurologist can choose stimulation parameters to optimally activate the pathways that best address the patient’s symptoms.
The Future is Collaborative
Vitek, Harel, and Johnson all believe that the Udall Center’s collaboration across several disciplines has been key to the innovations it has achieved.
“Together, we’ve figured out the neurological targets for certain symptoms” of Parkinson’s and how to treat them, Johnson says. “We’ve also learned how to avoid side effects of stimulation, such as mood changes and unwanted muscle contractions. We’ve learned where not to stimulate.” Together, they note that the ability to identify where to implant leads and which electrodes to activate for each individual patient has been a wonderful example of how collaborations across clinical and engineering programs can make a difference in patient outcomes.
Johnson and his team are now researching ways to determine optimized therapeutic DBS settings for patients before they come to a clinic so that they can be treated more quickly. Longer-term, patients with DBS may even have their devices “programmed” from their homes.
Vitek also believes that engineers can assist in the development of new lead designs for DBS. He notes that a few years back, medical technology companies introduced leads that allowed for directional stimulation. “Instead of a single circular band of metal, the lead is split into three segments,” Vitek says. With this design, “If I’m too close to an area I don’t want to stimulate, I can turn off the segment that’s facing that area.”
Vitek would like to see lead designs that offer even greater flexibility. “This is where bioengineers like Johnson and his team come into play,” he says. “They can look at the details of current flow and, working with industry, they can help innovate the designs of novel leads.” In other words, by “looking at all aspects of deep brain stimulation” with academic clinicians and engineers, med-tech firms can further improve the effectiveness of DBS therapies—and improve many more lives.



