Histology Core
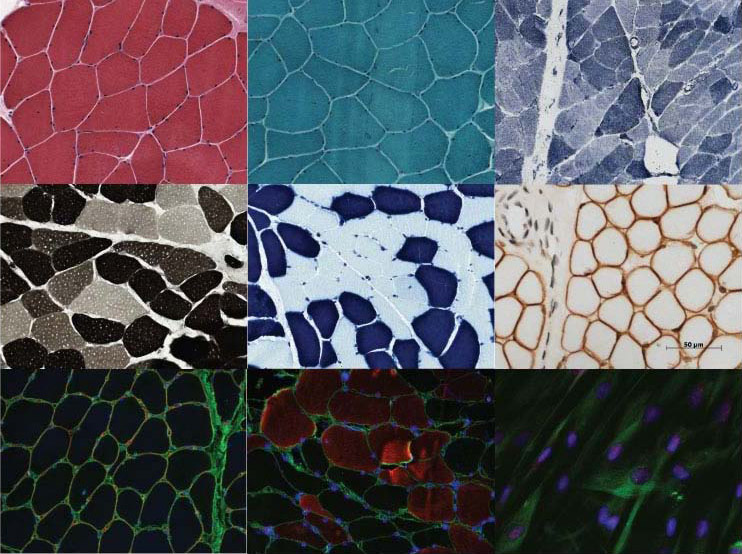
The histology core provides routine muscle histochemistry and light microscopy on frozen sections using the following stains: hematoxyln and eosin (H&E), PAS stain for glycogen, oil red O stain for lipid, Congo red, Gomori trichrome, ATPase (pH 4.3, 4.65 and 9.4), metachromatic ATPase, NADH, SDH, modified SDH, COX, α-GP, acid phosphatase, phosphorylase A, myoadenylate deaminase and nonspecific esterase. For most animal models, muscle is stained with H&E, Gomori trichrome, ATPase, SDH, and acid phosphatase, though additional stains can be performed as needed to characterize the specimen. Formalin-fixed, paraffin-embedded tissue is also routinely studied using H&E, Congo red and PAS stains, and will be available for individual projects as needed. Immunoperoxidase staining with monoclonal antibodies directed against spectrin, dystrophin-1 (rod domain between amino acids 1181 and 1388), dystrophin-2 (last 17 amino acids of carboxy terminus), dystrophin-3 (amino terminal exons 10-12), α-sarcoglycan, δ-sarcoglycan, β-sarcoglycan, γ-sarcoglycan, α-dystroglycan, caveolin, dysferlin, desmin, vimentin, laminin, lamin A/C and emerin will be utilized to identify potentially novel forms of disease. Immunofluorescence methods are also available for many anti-bodies. In addition, RNA FISH will be performed on human muscle samples to identify patients with possible novel forms of myotonic dystrophy.
Resources and Facilities
The histology core is located in the Wallin Medical Biosciences Building. Major histology equipment includes: Leica ASP 300S tissue processor that can handle 300 cassettes at one time for formalin fixation; Leica EG1150C/H tissue embedding station; Leica RM212RT microtome; Leica Auto-Stainer XL that is fully enclosed and allows for 20 different programs for various histological stains; a Leica CM 1900 cryostat for frozen sections; a Nuaire DF8524G, -80ºC ultralow freezer and a liquid nitrogen cryogenic tank.