Evidence Synthesis Program
The Evidence Synthesis Program is a collaboration of CLHSS and the Minnesota Evidence-Based Practice Center. It was created to address the need for rapid, high-quality evidence to inform clinical practice and guidelines.
We evaluate topic areas where evidence is emerging or where evidence gaps exist to inform and adapt clinical practice. Our skills and services include a range of reviews, such as systematic and scoping reviews and meta-analyses. Under appropriate circumstances, we perform rapid reviews that answer questions using a critical and rigorous but time-limited approach.
The Evidence Synthesis Program is led by Mary Butler, PhD, MBA and Josh Rhein, MD. Bronwyn Southwell, MD is the Integration Lead and Sallee Brandt, MPH is the project manager.
Work With Us
-
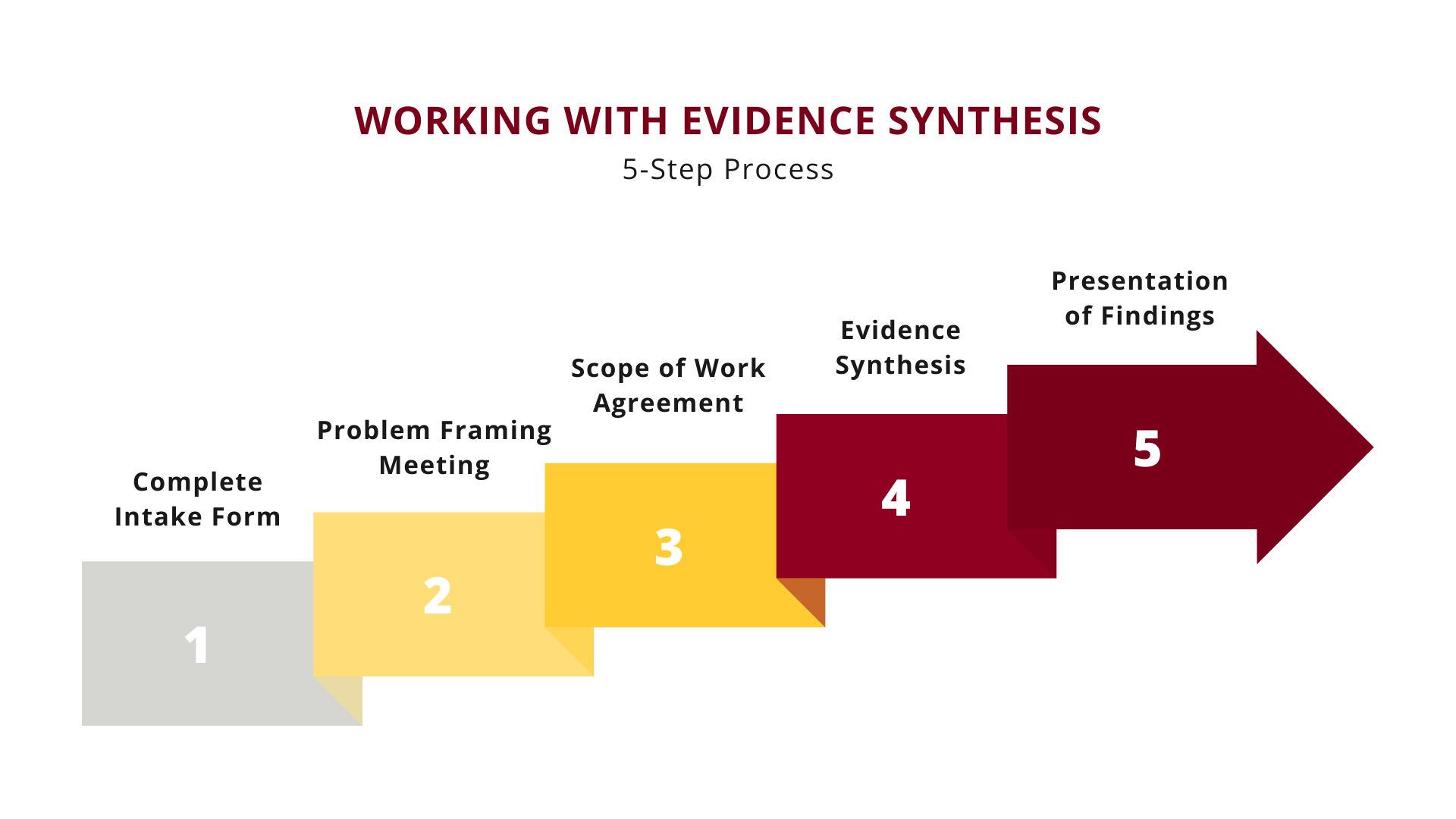
The process for requesting an Evidence Synthesis review begins with filling out the CLHSS Services Request Form. In this form, we ask questions about the problem or decisional dilemma you are facing, how you will use the information, and when you need it. We review each submission to determine its suitability for an evidence review.
-
If your request is suitable for a review, we then schedule a Problem Framing meeting to further clarify and define your research questions.
-
After the Problem Framing meeting, your request is once more evaluated to determine if the scope of work fits within our Program’s purview. If yes, a Scope of Work agreement is created, which defines the scope of work that the Evidence Synthesis Program will undertake to fulfill your needs.
-
Once the Scope of Work agreement has been approved, the work of synthesizing the evidence begins. Depending on the scope and complexity, project timelines will vary from weeks to months.
-
After the evidence review has been completed, we will schedule a meeting to present our findings. You will be provided with final, archivable documents for your use.
Evidence Synthesis People
Leadership
Mary Butler, PhD
Bronwyn Southwell, MD
Full Members
Josh Rhein, MD
Core Member
Project Manager, Sallee Brandt, MPH
Recent Evidence Synthesis Projects
Administration of Beta-lactam Antibiotics
Project Purpose
Different M Health Fairview Hospitals use different practices to administer beta-lactam antibiotics, specifically piperacillin-tazobactam. Some sites use an extended or prolonged infusion strategy, while others use a standard, intermittent infusion strategy. This variation is confusing for providers who move between hospitals.
The Evidence Synthesis Unit conducted this rapid review in response to a request from the M Health Fairview Pharmacy Department, who wanted to standardize the practice and ensure all patients are receiving the same quality of care.
Prolonged infusions have both theoretical benefits and potential drawbacks, making it unclear which infusion strategy is the most effective. In this project, we reviewed the appropriate dosage and use of prolonged versus standard infusions of piperacillin-tazobactam.
Results Summary
We found four recent systematic reviews and four high-quality randomized controlled trials that compared different infusion lengths and timing.
- Patients who received continuous, prolonged infusions were significantly more likely to be cured of their clinical conditions.
- The systematic reviews suggested that patients who received prolonged infusions had significantly less mortalities, while only one randomized controlled trial found a difference.
- There were conflicting findings on if prolonged infusions affected patients’ length of stay and their odds of adverse effects or microbiological cure.
- Prolonged infusions are possibly more effective than standard infusions.
We did not find any studies that compared different dosages of piperacillin-tazobactam.
Systematic Reviews
(Fawaz, 2020; Rhodes, 2018; Yang, 2016; Yang, 2015)
Randomized Controlled Trials
(Chongcharoenyanon, 2021; Lyu, 2018; Naiim, 2022; Yang, 2017)
Questions
Please contact Toyin Lamina, PhD, MPH: lamin019@umn.edu
Antibiotic Irrigations in Surgical Prophylaxis
Project Purpose
Antibiotic irrigations are utilized throughout the M Health Fairview System as part of surgical prophylaxis and are intended to help prevent infection by administering antibiotics prior to an operation. However, in many circumstances it is unclear how the benefits of irrigation compare to its risks.
The Evidence Synthesis Unit conducted this rapid review in response to a request from the M Health Fairview Pharmacy Department. The Pharmacy Department was interested in evaluating the use of irrigations in surgical prophylaxis to determine which specific procedures and patient populations it should be used for.
For this project, we conducted a literature search to find high-quality clinical guidelines that give recommendations on irrigation and topical antibiotics. Additionally, we conducted a review to determine if specific patient populations benefit from antibiotic irrigation.
Results Summary
We found three guidelines that were based on evidence from systematic reviews.
- The guidelines were from highly-regarded guideline development groups: the Center for Disease Control (CDC), National Institute for Health and Care Excellence (NICE), and World Health Organization (WHO).
- All three organizations agreed that antibiotic irrigation or antimicrobial agents like vancomycin powder are generally not recommended for surgical prophylaxis.
Our team performed a broad search of different patient groups before narrowing our review to adult and pediatric patients undergoing orthopedic surgery.
- One high-quality systematic review found that in most studies, administering vancomycin powder to adults did not significantly lower their odds of prosthetic joint infection (Wong, 2021).
- Two low-quality systematic reviews found that vancomycin powder significantly reduced patients’ odds of prosthetic joint and surgical site infections (Movassaghi, 2022; Kim, 2021).
- There were few randomized controlled trials on this topic to provide high-quality evidence.
- No research examined the harms of vancomycin powder.
Questions
Please contact Toyin Lamina, PhD, MPH: lamin019@umn.edu
Bactisure & Irrisept Wound Lavage in Elective Arthroplasty
Project Purpose
Total joint arthroplasty (TJA) is among the most common surgeries performed in the United States. The goal of arthroplasty is to improve function and quality of life, generally in older adults. Periprosthetic joint infection (PJI) occurs in up to 2% of arthroplasty recipients overall and is one of the most serious adverse events related to TJA, often with long-term consequences.
Wound irrigation (lavage) is a surgical wound washout process used during arthroplasty procedures to remove debris from the operative wound and reduce microbial contamination that can lead to infection. In primary arthroplasty, the main goals of surgical lavage are to prevent surgical site infection (SSI) and microbial biofilm development and aid visualization of the operative area.
The Evidence Synthesis unit conducted this rapid review in response to a request from a clinical working group interested in understanding two different wound lavage solutions and their comparative effectiveness in TJA, and revision arthroplasty in particular.
The two lavage options of interest were Bactisure® and Irrisept®. The Bactisure® Wound Lavage (BWL) system includes a 1000 ml bag of aqueous solution to remove debris from surgical wounds using pulsed (jet) lavage followed by saline, and is often used for revision arthroplasty surgeries. BWL was specifically designed to deconstruct and remove biofilm to make bacteria more susceptible to antibiotics. Irrisept®, a 0.05% chlorhexidine gluconate in sterile water jet lavage irrigation solution, has bactericidal and anti-biofilm properties and is used for lavage in orthopedic surgery, in addition to general surgery and urology.
Results Summary
- We found a striking absence of randomized trials on the comparative effectiveness of surgical lavage solutions used during elective arthroplasty surgeries. Large cohort studies with a concurrent comparison group are also absent.
- Based on clinical studies to date, there is insufficient clinical evidence to determine the efficacy or comparative effectiveness of Bactisure™ or Irrisept® wound lavage for the prevention or treatment of infections associated with total joint arthroplasty of the hip, knee or shoulder.
- The literature suggests that there remain great uncertainties around which specific agent or combinations thereof best prevents prosthetic joint infections or removes biofilm.
- Nonetheless, if Bactisure™ does have biofilm-busting capabilities that translate from in vitro to clinical settings, then Bactisure™ would likely be indicated for revision arthroplasty when infection (PJI) is present and known, provided the risk of adverse effects (e.g. cytotoxicity) is small.
- There are several limitations of this rapid review. Our searches focused on arthroplasty and specific irrigants, so we excluded evidence from other subspecialties, when identified.
Questions
Please contact Toyin Lamina, PhD, MPH: lamin019@umn.edu
Depression PROMIS Score Validity
Project Purpose
The Patient-Reported Outcomes Measurement Information System Global Health survey (PROMIS-GH) is a questionnaire used broadly across M Health Fairview to measure the outcomes of adult and pediatric patients with depression.
The Evidence Synthesis Unit conducted this rapid review in response to a request from a clinical working group interested in interpreting and incorporating the depression-specific measures/scores in PROMIS-GH into clinical practice.
This investigation into PROMIS-GH is part of the group’s larger work of looking at M Health Fairview’s continuum of care for patients with depression and creating a care maplet.
For this project, our team examined the minimally important changes (MICs) in PROMIS-GH scores in patients with chronic pain, anxiety, post-traumatic stress disorder, depression, or other mental health conditions.
Results Summary
- Current research literature supports reporting the 10-item PROMIS-GH score as two subscores: PROMIS Physical Health (PROMIS-PH) and PROMIS Mental Health (PROMIS-MH).
- While a number of studies calculated MIC values for other PROMIS scales, none calculated an MIC for the PROMIS-MH subscore.
- No studies focused on patients with depression or used PROMIS-GH exclusively for mental health and chronic pain patients.
Questions
Please contact Toyin Lamina, PhD, MPH: lamin019@umn.edu
Food is Medicine
Project Purpose
Food is Medicine (FIM) is an initiative through the Center for Community Health Equity that aims to reduce food insecurity and chronic diet related conditions among patients and improve health outcomes. The initiative also aims to reduce burden on providers and the total cost of care for the health system.
FIM programming has been implemented across M Health Fairview for a number of years. The programming includes food distribution, monthly payments for groceries, classes to improve food skills, and more.
The Evidence Synthesis Unit conducted this review in response to a request from M Health Fairview seeking an investigation into the impact of FIM programming as well as a review of other food insecurity interventions that could potentially be implemented.
Results Summary
A large number of studies on food interventions were identified. The three main types of interventions discussed were referrals to community organizations, food or food vouchers, and a combination of referrals, food, and/or nutrition education.
- Food/food vouchers significantly reduced food insecurity both alone and when combined with nutrition education.
- A web screening tool that referred patients to various agencies also reduced food insecurity (Hassan, 2015).
- While there were mixed results on clinical outcomes, many interventions involving food/food vouchers and food/food vouchers with nutrition education improved patients’ BMI and blood glucose.
- One intervention that involved screening and referring pregnant patients to a food resources program improved their blood pressure but did not have a significant effect on their blood glucose (Morales, 2016).
- The effects of interventions on healthcare utilization and costs were also mixed.
- Patients enrolled in a meal delivery program used emergency services significantly less (Berkowitz, 2018). Similarly, children given a combination of free formula, referrals, and nutrition education were more likely to seek preventative care (Beck, 2014).
- In other studies, patients receiving combinations of different interventions did not have significantly different healthcare utilization. One group that received referrals, food vouchers, and nutrition education even had lower numbers of patients attending follow-up appointments (Blistein, 2021).
Research on food security interventions varies greatly in terms of the intervention applied, population studied, data collected, and outcomes measured. Because we did not assess the quality of the literature, we can’t assess the strength of the association between these interventions and their outcomes.
The Food is Medicine team will use the findings of the Evidence Synthesis unit to design future programs and evaluations, in collaboration with CLHSS’s Practice Based Research Network.
Questions
Please contact Toyin Lamina, PhD, MPH: lamin019@umn.edu
Guidelines for Prescribing in Acute Sinusitis
Project Purpose
The Evidence Synthesis unit conducted this rapid review in response to a request from a clinical working group interested in understanding antibiotic treatment recommendations for acute sinusitis.
The working group was most familiar with the guidelines previously published by the Infectious Diseases Society of America (IDSA) and Institute for Clinical Systems Improvement (ICSI). However, both of these guidelines were outdated, and their recommendations conflicted with one another.
For this project, we assessed the quality of current (2018-present) clinical practice guidelines providing recommendations on antibiotic treatment for acute sinusitis.
Results Summary
One high quality and current clinical practice guideline was identified in our review - the European Position on Rhinosinusitis and Nasal Polyps 2020 (EPOS2020). The AGREE-II tool was used to assess guideline quality.
A summary of the EPOS2020 guideline for treatment of acute sinusitis:
- The EPOS2020 guideline included recommendations based on three distinct types of acute rhinosinusitis (ARS): acute viral rhinosinusitis, acute post-viral rhinosinusitis, and acute bacterial rhinosinusitis.
- Antibiotics were recommended for only adults diagnosed with acute bacterial rhinosinusitis.
- From limited evidence, it appears beta-lactams are effective and fluoroquinolone is not.
- There was not enough data to make an antibiotic recommendation for children with acute bacterial rhinosinusitis.
We carried out further work to identify any randomized controlled trials using antibiotics to treat acute sinusitis published after the EPOS2020 publication (January 2019). Ten additional studies were identified and shared with the working group.
Questions
Please contact Toyin Lamina, PhD, MPH: lamin019@umn.edu
Mammography Screening in Average-Risk Women
Project Purpose
Breast cancer is the most common cancer in women in the United States, and mammography is a crucial part of a comprehensive breast cancer surveillance strategy. Mammography screening may be associated with reduced breast cancer mortality but can also cause harm.
The Evidence Synthesis unit conducted a rapid review in response to a request from a clinical working group interested in understanding the quality of clinical practice guidelines providing recommendations on the frequency and interval of mammography screening.
There are important differences in the mammography screening interval and frequency recommendations between guidelines, resulting in variations in practice by different providers and specialties within the organization. The requesting group desired to standardize practice as much as possible.
Results Summary
Seven different US-based clinical practice guidelines published from 2012 to 2022 were assessed using the Appraisal of Guidelines for Research and Evaluation II (AGREE-II) tool.
- The U.S. Preventive Services Task Force (USPSTF) 2016 guideline was investigated further because it was assessed to be a high quality guideline, and commonly utilized by the requesting organization.
- The American College of Radiology (ACR) / Society of Breast Cancer Imaging (SBI) 2021 guideline was also investigated further. It was assessed to be of moderate quality, but was more current and also commonly utilized by the requesting organization.
The differences between the USPSTF 2016 and ACR/SBI 2021 guidelines were investigated:
- ACR/SBI’s recommendation for screening is that it should begin at 40 and continue annually.
- USPSTF recommends using shared decision making for mammography screening for ages 40-49. They recommend biennial screening starting at 50.
- Both groups insist on communication between women and their provider (shared-decision making).
In conclusion, It is difficult to uncover which guideline is superior between USPSTF and ACR/SBI due to the differences in guideline development processes and modeling assumptions. However, both USPSTF and ACR/SBI stress the importance of shared decision-making.
Questions
Please contact Toyin Lamina, PhD, MPH: lamin019@umn.edu
Rapid Review on the Impact of the SEP-1 Bundle on Patient Outcomes
Project Purpose
The Evidence Synthesis unit conducted this rapid review in response to a request from a clinical working group interested in understanding which aspects of the Severe Sepsis and Septic Shock Management Bundle (SEP-1) were most important for patient outcomes.
SEP-1 mandates the administration of a bundle that carefully prescribes how patients with severe sepsis and septic shock must be treated in the early phases of care. The 1-hour bundle is composed of the following five elements: measuring the lactate level, obtaining blood culture prior to administration of antibiotics, administering broad-spectrum antibiotics, beginning rapid administration of 30 mL/kg crystalloid fluid for hypotension or lactate ≥4 mmol/L, and administering vasopressors if the patient is hypotensive during or after fluid resuscitation.
For this project, we developed a broad synopsis of the available literature through an evidence map on SEP-1 compliance, fluid resuscitation, and antibiotic administration in sepsis management. We then conducted a rapid review of the literature published over the last five years.
Results Summary
Our review produced several key points:
- Recent evidence on sepsis patient management is limited, largely observational (non-randomized), and generally low quality.
- The field needs high-quality RCT evidence to better inform patient care.
- The evidence base for fluid resuscitation (volume) appears wide and includes several systematic reviews.
- No recent systematic reviews address the question of initial timing of fluid resuscitation, representing a research gap in this area.
- An updated review on antibiotics de-escalation is needed, as the most recent one is more than five years old.
- Little to no research has directly addressed harms of antibiotic administration for patients at low risk of infection or suspected sepsis, representing another research gap in this area.
- Key points: care bundles such as SEP-1 may improve survival; effects of fluid volume comparisons are mixed; early antibiotic administration (within 1 hour) showed no or equivocal benefit compared to later antibiotic initiation; antibiotic de-escalation showed no detrimental effect for mortality.
Questions?
Please contact Toyin Lamina, PhD, MPH: lamin019@umn.edu
Education
We have a free educational module for anyone interested in evidence-based care, Evidence Synthesis 101.
Funded Projects
M-PALS Collaborative
The MN-EPC is developing a set of clinical practice guidelines for pain management in abdominal laparoscopic patients that will be implemented and evaluated.