First Minnesota brain tumor patient enrolled in study to evaluate effect of laser ablation on quality of life
Using a novel magnetic resonance imaging (MRI)-guided laser technology, neurosurgeons at the University of Minnesota have successfully treated a tumor located deep inside a patient’s brain. This patient was enrolled in the LAANTERN (Laser Ablation of Abnormal Neurological Tissue using Robotic NeuroBlate System) study, a multi-center effort designed to evaluate how this precision laser treatment affects the patient’s quality of life.
“Patients with malignant brain tumors located deep in the brain are typically not considered optimal candidates for surgery, because of the extensive amount of healthy brain tissue that can be damaged during an operation,” said Clark Chen, MD, PhD, Head of the U of M’s Neurosurgery Department and Principal Investigator in the LAANTERN study. “As a result, overall survival and quality of life for these patients are poor. By applying this next-generation, precision laser treatment, however, we can help patients who would otherwise be considered inoperable.”
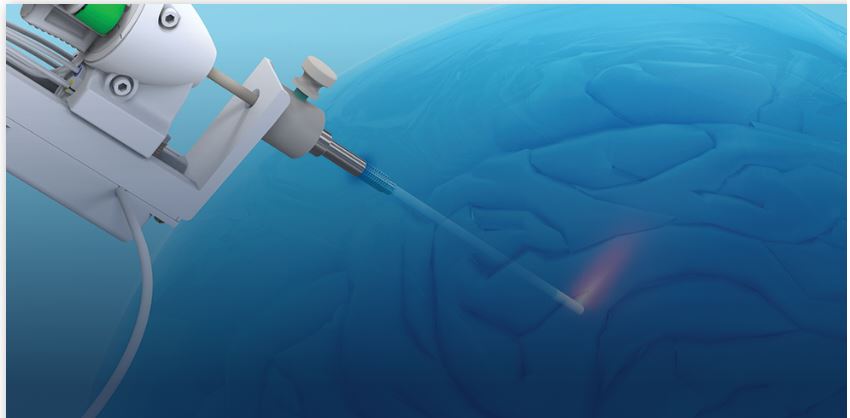
The actual laser procedure requires a small, pea-sized skin incision and a similarly sized opening in the skull. The laser probe is inserted in this opening directly into the tumor and used to destroy the cancer cells, with minimal impact to nearby healthy brain tissue. The laser treatment requires real-time MRI monitoring and a dedicated intra-operative MRI team. It cannot be performed in a conventional operating room.

U of M LAANTERN Clinical Trial lead Clark Chen, MD, PhD, Neurosurgery Department Head
“I have treated many brain tumor patients with this minimally invasive laser procedure and my experience is that the treatment significantly improved their quality of life,” Chen noted. “Through LAANTERN, we will determine whether my experience holds true for all enrolled patients.”
As a part of the study, participating physicians will measure enrolled patients’ quality of life factors such as physical, social, emotional and functional well-being, in addition to how well the symptoms of disease are being controlled. The study hopes to enroll a total of 1,000 patients across all participating sites.
“By understanding how our treatment impacts patients’ lives, we can better serve our brain tumor patients and develop the interventions necessary for improving their quality of life,” said Chen. “Unfortunately, physicians sometimes narrowly focus on survival of cancer patients without careful consideration for the quality of that survival. This study represents a necessary step toward where we need to be in 2018 in terms of oncology care.”
Genuine multi-disciplinary effort is required in the execution of this landmark study, according to Chen. He will be joined by department colleagues Matthew Hunt, MD; Daniel Guillaume, MD, MS; Michael Park, MD, PhD; and Bob McGovern, MD; neuro-oncologist Elizabeth Neil, MD; oncologist Emil Lou, MD, PhD; and Evidio Domingo-Musibay, MD; as well as radiation oncologists Kathryn Dusenbery, MD; and Christopher Wilke, MD, PhD.
“We are proud to support this advanced form of minimally invasive surgery and define the next generation of neurosurgical care, with the goal of improving the quality of life for our brain tumor patients,” said Chen.
The LAANTERN study is funded by NeuroBlateÒ System manufacturer, Plymouth, MN-based Monteris Medical Corporation, and is designed to collect patient outcome data for a therapeutic technique that uses laser energy to destroy brain tumor tissues.
Monteris Medical is a privately held company based in Plymouth, MN, that develops devices for minimally invasive, MRI-guided neurosurgery. Monteris markets the NeuroBlate System for controlled, volumetric ablation of many forms of abnormal brain growth, including brain tumors. Monteris also offers various stereotactic anchoring devices for image-guided trajectory alignment, and the AtamA™ Stabilization System for MRI-based procedures requiring head fixation.
To learn more about laser thermal ablation and the LAANTERN trial, contact the University of Minnesota Department of Neurosurgery at 612-624-6666, or email Nonyelum Harcourt at harco002@umn.edu.