Stroke, Brain Injury, and Stem Cell Lab
Our lab’s research has primarily focused on treating ischemic stroke and traumatic brain injury (TBI) with neuro-regenerative therapies, using either stem cells or direct reprogramming techniques. Recovery after stroke or TBI is limited in the human adult brain due to minimal or no regeneration of lost neurons. Over the past 10 years, more work has been done on the potential use of neural stem cells to regenerate neurons in this setting. We focus on direct manipulation or reprogramming of non-neuronal cell populations through viral transduction of various neurogenic transcription factors. We have been able to reprogram non-neuronal cells into neurons both in vitro and in vivo. Interestingly, we found a significant difference in the neuronal phenotype generated and maturation of neurons between the striatum and cortex suggesting a significant environmental influence. Our study was one of the first to demonstrate direct reprogramming, in vivo, in a rodent brain. More recently, we have been focusing on using an adeno-associated virus and have found both a reprogramming and a neuroprotective effect in stroke and TBI.
Neuroinflammation
Our lab team has also used various animal models of stroke to study different interventions aimed at reversing the cascade of events that lead to cell death in the penumbra. We initially focused on apoptosis and discovered that a bile acid with anti-apoptotic properties minimized cell death in the penumbra and resulted in improved outcomes when given within two hours after stroke. Interested in extending this therapeutic window, we began testing the neuroprotective effect of administering human umbilical cord blood stem cells (HUCBSCs) intravenously. Surprisingly, we discovered a robust neuroprotective effect when HUCBSCs were administered after stroke, even out to 48 hours. More recently, we have focused on the mechanistic action responsible for this protective effect in both stroke and TBI and have found that HUCBSCs act directly on the heightened inflammatory state after the injury, returning it to near baseline levels.
Neuroplasticity
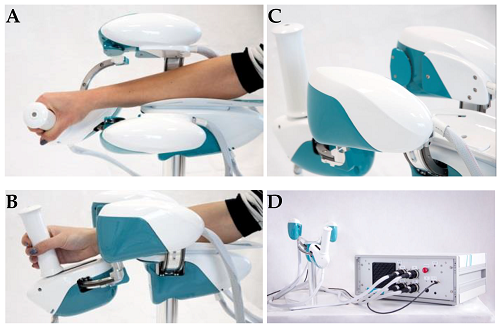
Neurons in regions unaffected by a stroke may take on roles previously performed by those destroyed by it. This neuroplasticity can potentially be induced in humans by inhibiting the normal contralateral inhibition of the area around the infarction using transcranial magnetic stimulation (TMS). This effect is then enhanced through repetitive activity involving the ipsilateral (side of the stroke) hemisphere of the brain. Our initial pilot study examined patients who had suffered a stroke and had arm weakness or paralysis. Using noninvasive brain-computer interface, we discovered that these patients could be trained to perform a single arm action in a 3D, virtual reality environment. In addition, patients with basal ganglia strokes performed much better than patients with cortical strokes. Currently, we are using animal models of stroke and treating the condition with neurorobotic tools, such as Wristbot (pictured above left) and KinArm, to better understand the pathophysiology of neuroplasticity following stroke. Our long-term vision is that understanding neuroplasticity better can help improve treatments such as transcranial magnetic stimulation.
Therapeutic application of umbilical cord blood stem cells (UCBSCs) for treatment of stroke & TBI
- Translational studies of UCBSCs focused on advancing to human clinical trial for stroke treatment
- Mechanistic studies in animal models of stroke and TBI to understand pathophysiology
- Neuroinflammatory studies to better understand influence of UCBSCs on neuroinflammation
Reprogramming in vivo astrocytes for stroke/TBI treatment
- In vitro and in vivo animal studies to demonstrate reprogramming of reactive astrocytes into neurons after stroke/TBI
- Mechanistic studies in animal models of stroke/TBI to better understand therapeutic efficacy of reprogramming reactive astrocytes into neurons
- Translational studies of in vivo reprogramming in stroke with a focus on advancing to human clinical trial
Pathophysiology of neuroplasticity to enhance neuro-rehabilitative therapy of stroke
- Animal studies of stroke to better understand pathophysiology of neuroplasticity
- Mechanistic studies in animal models of stroke to better understand pathophysiology behind restorative effects of neurorobotic devices (e.g., WristBot, KimArm).
- Characterizing the Neuroinflammation Associated with Sequential TBI in a Rodent Model
MN Office of Higher Education - Reprogramming Astrocytes into Neurons to Provide Therapeutic Benefit in TBI
MN Office of Higher Education - Harnessing Exosomes as a Biomarker and Therapeutic Approach to Traumatic Brain Injury
MN Office of Higher Education - Generating Exoenic Hippocampal Neural Cells via Blastocyte Complementation for Transplantation in Alzheimer’s Disease
NIH-NIDDK - Repairing the Brain after Stroke-Neuroregenerative Therapy in a Canine Model of Stroke
NIH - Stem Cells for Treating Acute Stroke
Saneron Ccel Therapeutics, Inc/NIH – NINDS - CREST-2 Clinical Trial Coordinating Center
Mayo Clinic Jacksonville/NIH (Prime) - Vorus Medical I-Corps Submission
NSF Innovation Corps (NSF 18-515) - Extracorporeal Filtration of Subarachnoid Hemmorrhage via Spinal Catheter
Minnetronix Medical, Inc. - Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial – Hemodynamics (CREST-H)
Mayo Clinic Jacksonville/Columbia University Medical Center/NIH (Prime) - Novel Highly Regenerative and Scalable Progenitor Cell Exosomes for Treating Stroke
AgeX Therapeutics, Inc. - Automation of Extracorporeal Filtration of Subarachnoid Hemorrhage Via Spinal Catheter
Minnetronix, Inc / NIH (Prime)
- Johnson N, You A, Carey J, Van de Winckel A, Grande A, and He B. Improving Motor Recovery after Stroke by Combined rTMS and BCI Training. Poster Presentation. Sixth International Brain-Computer Interface (BCI) Meeting, Monterey, California, May 30 - June 3, 2016
- Stone L, Xiao F, Rotshafer J, Nan Z, Juliano M, Sanberg C, Sanberg P, Kuzmin-Nichols N, Grande A, Cheeran M, Low, W. (2016) Amelioration of Ischemic Brain Injury in Rats with Human Umbilical Cord Blood Stem Cells: Mechanisms of Action. Cell Transplantation. In Press
- Johnson N, You A, Carey J, Van de Winckel A, Grande A, and He B. Improving Motor Recovery after Stroke by Combined rTMS and BCI Training. Oral Presentation. Minnesota Neuromodulation Symposium, Highlight Talk, Minneapolis, Minnesota, April 14 - 15, 2016
- Stone L, Grande A, Low W. (2013) Neural Repair and Neuroprotection with Stem Cells in Ischemic Stroke. Brain Sciences 3(2): 599-614
- Nakafuku M, Grande A. (2013) Neurogenesis in the Damaged Mammalian Brain, Chapter 29, pgs 551-608. In John Rubenstein and Pasko Rakic, eds. Patterning and Cell Type Specification in the Developing CNS and PNS: Comprehensive Developmental Neuroscience
- Lopez-Juarez A, Howard J, Ullom K, Howard L, Grande A, Pardo A, Waclaw R, Sun Y, Yang D, Kuan C, Campbell K, Nakafuku M. (2013) Gsx2 controls region-specific activation of neural stem cells and injury-induced neurogenesis in the adult subventricular zone. Genes Dev 27(11): 1272-87. PMID: 23723414
- Grande A, Sumiyoshi K, Lopez-Juarez L, Howard J, Sakthivel B, Aronow B, Campbell K, Nakafuku M. (2013) Environmental Impact on Direct Neuronal Reprogramming In Vivo in the Adult Brain. Nature Communications 4:2373. PMID:23974433
- Nakafuku M, Nagao M, Grande A, Cancelliere A. (2008) Revisiting Neural Stem Cell Identity. Proc Natl Acad Sci. 105(3): 829-30. PMID: 18195367, Elsevier/Academic Press. http://dx.doi.org/10.1016B978-0-12-397265-1.00074-5
- Nan Z, Grande A, Sanberg C, Sanberg P, Low W. (2005) Infusion of Human Umbilical Cord Blood Ameliorates Neurologic Deficits in Rats with Hemorrhagic Brain Injury. Ann. N.Y. Acad. Sci. 1049:84-96. PMID: 15965109
- Rodrigues C, Spellman S, Sola S, Grande A, Cheryle L, Low W, Steer C. (2002) Neuroprotection by Bile Acid in an Acute Stroke Model of Rat. J. Cerebral Blood Flow. 22: 463-471. PMID: 119195117
- U of M Center for Magnetic Resonance Research, a world-renowned research facility known for its pioneering work in ultrahigh-field MRI: Pierre-François Van de Moortele, MD, PhD; Jan Zimmermann, PhD; Wei Chen, PhD
- Brain Injury Research Consortium (Stroke/TBI): Andrew Grande, MD; Walter Low, PhD; Maxim Cheeran, MVSc, PhD; Jesse Williams, PhD; Timothy Ebner, PhD
- Brain Aneurysm Research Consortium: Andrew Grande, MD; Brenda Ogle, PhD; Patrick Alford, PhD; Victor Barocas, PhD; Omid Amili, Pierre-François Van de Moortele, MD, PhD; Bharathi Jagadeesan, MD; Filippo Coletti, PhD
- Neurorobotics Consortium: Andrew Grande, MD; Ann Van de Winckel, PhD, MSPT, PT; Timothy Kowalewski, PhD; and Jürgen Konczak, PhD
- Trigeminal Neuralgia Consortium: Donald Nixdorf, DDS; Andrew Grande, MD; Kellen Mulford, PhD, Sean Moen, Pierre-François Van de Moortele, Stephen Haines, MD
Directors
Medical/Graduate/Masters Students
- Tony Larson
- Isaac Clark
- Alexander Roman
- Sam Schulz
Researchers
- Maple Shiao, MD, PhD
- Venkat Krishna, PhD
- Anala Shetty, MS
- Dilmareth Natera
Undergraduates
- Kevin Sun
- Zach Roush
Managers
- Sean Moen
Past Members
- Nan (Crusoe) Zhenhong, M.D., currently a scientist at the University of Michigan
- Mario Julian, currently a medical student at the New York Medical College
- Joshua Lim, MD
- Nicholas Dick
- Zach Rollins
- Quincy Rudman
- Morgan Forgette
- Kyle Schaible
- Matthew Chrostek
- Nicole Emmitt
- Emily Fellows
- Andrew Crane, PhD
- Aleta Steevens, PhD
- Susanne Var, PhD
- Swathi Radha, MS
- Nikolas Toman
- Grant Badger