U of M research team to use 5-year, $2.2M NIH grant to investigate improving deep brain stimulation outcomes for epilepsy patients
Epilepsy is a common disorder in which neural activity in the brain is disturbed, causing seizures. While there are many different reasons someone can develop epilepsy, most of the time, seizures can be managed completely with medication. In about one-third of patients, however, medications do not control the seizures. At that point, the physician managing the person’s epilepsy care – usually a neurologist — may recommend a neurosurgical procedure known as deep brain stimulation (DBS).
The FDA approved the DBS settings used to help treat epilepsy in 2010, based on the results of the SANTE (Stimulation of the Anterior Nucleus of the Thalamus for Epilepsy) trial. During the procedure, the neurosurgeon implants electrodes in the patient’s thalamus (a region of the brain widely connected to other areas that may be responsible for promoting seizure activity) and a neurostimulator in their chest, which is connected to the electrodes. The stimulator is then programmed by the patient’s care team to the settings determined by the SANTE trial.

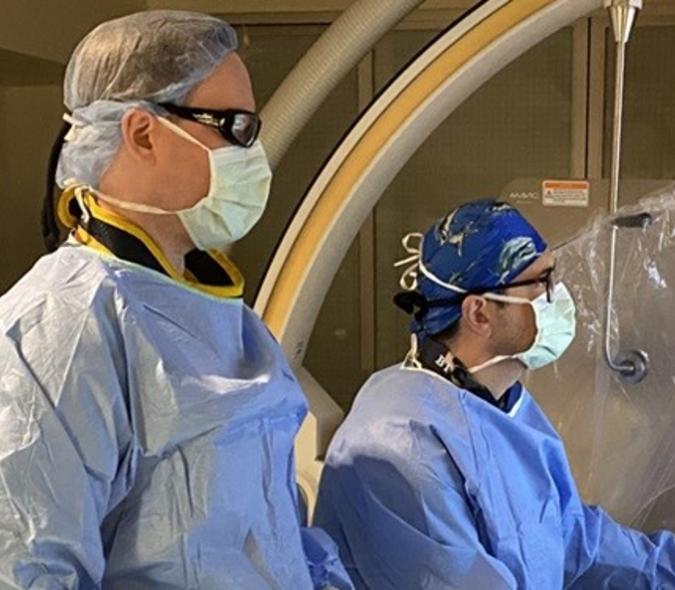
It is those settings that interest a multidisciplinary U of M research team. “One of the big issues with neuromodulation for epilepsy is determining the best stimulation parameters,” said neurosurgeon Robert McGovern (pictured here), MD. “You turn the stimulator on and use the settings that were shown to work in FDA-approved trials. That’s it. With movement disorders, our neurologists who program stimulators can change the settings to immediately see how it improves the patient’s ability to move. You can’t do that with epilepsy because of the sporadic nature of the seizures.”
Epilepsy research group
McGovern is part of an epilepsy research group at the U that includes Tay Netoff, PhD, from Biomedical Engineering. “Tay created some algorithms designed to optimize stimulation parameters in other neurological conditions,” said McGovern. “For example, he works with Dr. David Darrow in the Neurosurgery Department on spinal cord stimulation and in that context, has developed stimulation algorithms to help people regain movement and to restore different functions affected by the spinal cord injury.”
The research group recognized the potential of using Netoff’s algorithms to optimize deep brain stimulation settings for epilepsy. They developed a grant proposal and were awarded a five-year, $2.2 million National Institutes of Health UO1 grant, which will be used to create an experimental clinical trial managed by the U of M.
McGovern and Netoff are co-Principal Investigators on the grant. Other members of the team include Tom Henry, MD; Ilo Leppik, MD; Thaddeus Walczak, MD, and Yoon-Hee Cha, MD, from the Neurology Department, and Tom Murray, PhD, from the School of Public Health.
The goal is to create patient-specific stimulation setting optimization for those electing to have DBS to control their epilepsy. The research team plans to enroll 20 patients in the trial.
Closing the gap
“We’re interested in trying to close the gap in neuromodulation surgery that gets us closer to seizure freedom,” said McGovern. “People are hesitant to have traditional resection surgery and are more willing to go through the DBS procedure because it’s seen as minimally invasive. Improving the outcomes of that procedure is an important goal.”
Netoff, who has been studying epilepsy throughout his career, agrees, and is using an elegant solution for identifying additional settings. “Bayesian optimization is used extensively in engineering when you want to make something better, especially when you’re managing numerous parameters,” he said. “It solved a lot of our problems related to quantifying what we had to measure regarding patient response to the DBS therapy.”
Finding biomarkers
The idea is to find biomarkers – signals from the patient’s brain – that can help the research team optimize the stimulation settings for each person, according to McGovern. “Can we find the settings that minimize specific activity in their thalamus,” he said. “We want to use Tay’s algorithms to efficiently determine the best settings and to understand how activity in the thalamus changes.”

Netoff (pictured here) uses a subset of artificial intelligence called machine learning to develop his algorithms. “We’re creating a model of how the brain responds to different stimulation settings and then using it to suggest new settings,” he explained. “Just like Netflix uses algorithms to suggest a new movie based on one you just watched.”
Homing in
The team is using a Medtronic neuromodulation device that can both stimulate and record from the brain. “That enables us to isolate a number of settings and see which ones quiet the brain during a seizure,” Netoff said. “We’re using 9 different settings in the clinic to make our initial foray into how the brain responds and are approved for testing a total of 20. Hopefully, we’ll home in on a setting that benefits each patient.”
The first year of the study, the team is working on refining the algorithms. “We’re also testing patients in the clinic to make sure we can record the signal we want and to test as many settings as possible,” said McGovern. “Starting next fall, we’ll enroll people in the actual trial, which would send them home with a patient-specific, optimized setting to see how that changes the activity in the thalamus and what impact it has on reducing their seizures.”
Netoff emphasizes that the team doesn’t have a solution, they’re testing an idea. “It’s plausible and we hope it works,” he said. “We’re trying to make therapies like DBS more accessible, flexible, and patient-centric.”