Predicting brain aneurysm growth and rupture focus of significant new research grant
A long-term relationship between U of M colleagues Andrew Grande, MD, in the Neurosurgery Department, and Patrick Alford, PhD, of Biomedical Engineering, has helped launch a National Institutes of Health (NIH) RO1* grant titled, Role of mechanical heterogeneity in cerebral aneurysm growth and rupture. The award just received its first year of funding.
The five-year grant has three Principal Investigators who are led by Alford and include Grande and Victor Barocas, MS, PhD, of Biomedical Engineering. The research builds on previously published work done in collaboration with Elizabeth Shih, PhD; and Colleen Witzenburg, PhD, University of Wisconsin-Madison. This article explains the novel approach, first developed by Witzenburg and Barocas, that the team is using to help determine whether an aneurysm will rupture.

According to the Brain Aneurysm Foundation, an estimated 6.7 million people in the United States have an unruptured brain aneurysm (1 in 50 people). About 30,000 people in the country suffer a brain aneurysm rupture each year. That translates to roughly 1 rupture every 18 minutes. “Many of those people will die before they get to a hospital,” said Grande (pictured here).
It wasn’t that long ago that aneurysms were rarely found until they ruptured, according to Alford. “With today’s imaging technology,” he added, “they’re being found more often before that happens.”
It poses a dilemma for physicians – telling patients that they have a brain aneurysm, while admitting there is no way of predicting whether treatment (surgery) or no treatment (observation) is the best approach. “What our team wants to do is stratify the likelihood of rupture … to understand which aneurysms are most likely to rupture and require preventive surgery,” said Alford. “We want to determine when the risk of doing surgery is higher than that of the aneurysm rupturing.”
An international study seemed to indicate that the best predictor of rupture risk is the aneurysm’s size, according to Grande. “In the study, providers noted patients with aneurysms that should be treated or observed,” he said. “The ones that were chosen for observation were watched for five years. During that time, the incidence of rupture was zero for an aneurysm of less than 7 millimeters in size coming off the carotid artery. That sounds like such aneurysms should just be watched. But we see many patients with ruptured aneurysms of less than 4 millimeters.”

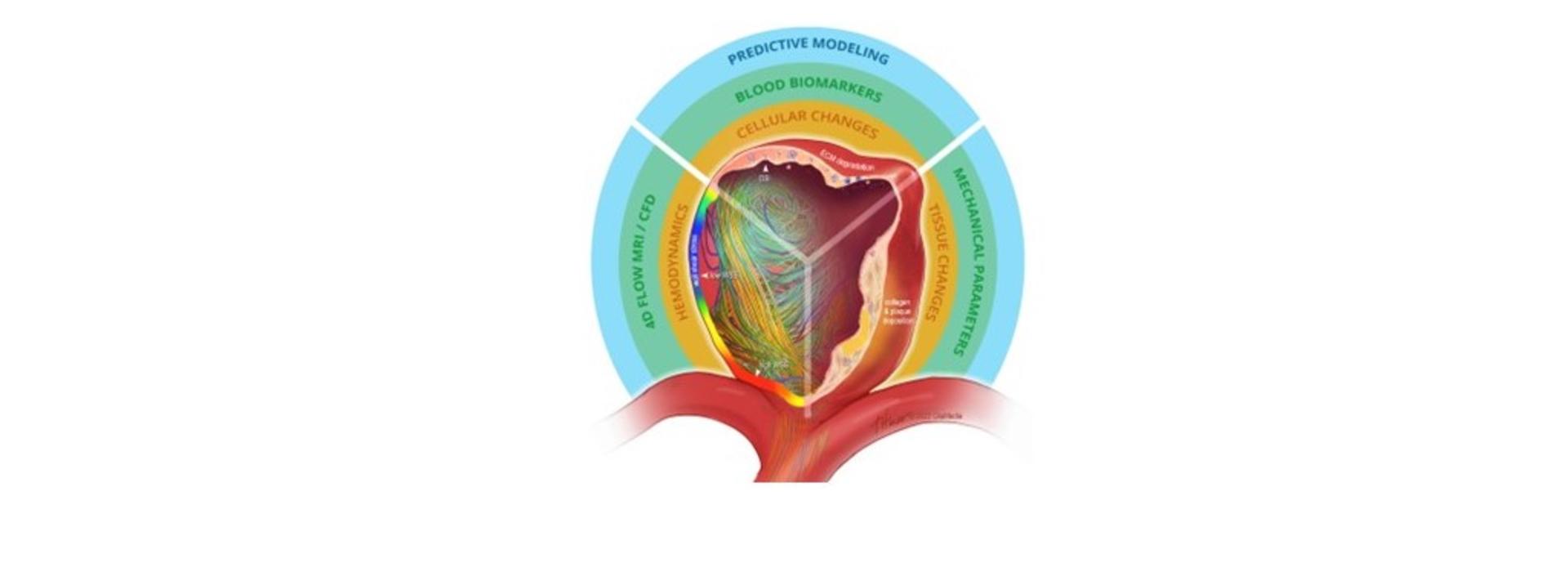
The research team’s goal is to eventually create clinically relevant stratifications that can guide a physician in making well-informed decisions about treating a patient’s aneurysm. “No one really knows why an aneurysm will rupture,” said Alford (pictured here). “We know that its wall becomes weak enough to no longer bear the pressure inside it and it bursts. Beyond that, it’s not like you can take a snapshot of these things and figure out their failure properties. What we want to do is first characterize what it takes to fail, which builds on our prior experiments, and second, be able to predict rupture based on things we can measure clinically.”
The team wants to learn as much as it can about the properties that lead to a ruptured aneurysm, take that data, and then use computational algorithms to develop a better predictive model. “It would help me as a clinician determine the risk of rupture,” said Grande. “It would help me talk to the patient about treating or not treating.”

Grande provides the clinical context for the RO1 team; Alford and Barocas (pictured here) provide the mathematical and computational expertise. For their earlier work, some members of the team used MRI imaging analysis to better understand the flow dynamics of patients aneurysms. “And when I remove an aneurysm, we examine its tissue’s mechanical properties at the cellular level,” he noted.
Some of the tissue the team works with is from unruptured aneurysms that Grande thought would be best removed. “We’re trying to characterize lower failure strength in these aneurysms,” said Alford. “Then we mechanically fail the tissue to find out how much stress it takes to do that and where it fails. That’s important.”
Grande, Alford, and Barocas are part of a unique group at the U of M known as the Brain Aneurysm Research Consortium. “This first RO1 builds on the expertise of the entire group,” said Grande. “The research we’re doing here about brain aneurysms is exciting — there are only a handful of other institutions doing similar work.”
*The Research Project Grant or RO1 is the original and historically oldest grant mechanism used by NIH. The R01 provides support for health-related research and development based on the mission of the National Institutes of Health.