DoS Health Outcomes Research Support
Key Takeaways
The Department encourages and supports outcomes based research via local and national databases. The Department provides access, expert consultation, and support to navigate the administrative and regulatory access requirements. There are research support staff to support faculty beginning to end in their research.
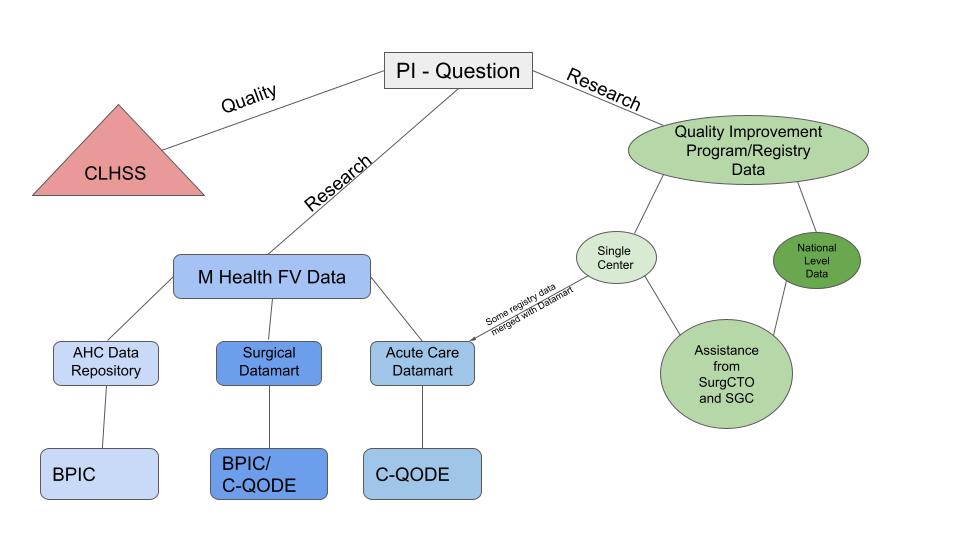
What data sources are available?
- DoS Data Sources (Datamarts)
- Acute Care Datamart
- Surgical Datamart (under construction)
- Medical School Data Source
- AHC-IE and CDR - Clinical Data Repository
- Available via BPIC (CTSI)
- Clinical Trial Feasibility and Recruitment
- Available via BPIC (CTSI)
- AHC-IE and CDR - Clinical Data Repository
- M Health FV, National, International Data Sources
- QA/QI Registries - QIPs
How do I gain access to the data?
Data Access Process Map
What approvals might I need to use the data?
- IRB approval
- Resource Review Process
- Unfunded Agreements (do not sign yourself!)
- Data Use (DUA)
- Material Transfer (MTARF)
- Confidentiality or Non-Disclosure Agreements (CDA/NDA)
The Surgery Clinical Trials Office (SurgCTO) will assist you in determining what agreements or approvals are required to gain access and conduct your research. Contact your division-assigned Program Manager about your project needs. You may also reach out directly to the general SurgCTO email at surgcto@umn.edu.
Are there experts who can help with design and analysis?
- BPIC: Best Practice Integrated Informatics Core
BPIC is part of the Clinical Translational Science Institute
To request a consultation, please complete BPIC’s Informatics Consultation Request Form
- C-QODE: Center for Clinical Quality and Outcomes Evaluation and Discovery
C-QODE is a DOS and Dean's office resource led by Drs. Tignanelli and Marmor.
If you have questions on C-QODE support, please contact: Alicia Herman - alicia@umn.edu or complete their Data Request Form.
- CLHSS: Center for Learning Health Systems Science
CLHSS is a collaboration between the Medical School and the School of Public Health focused on rapid QA/QI project integration. This is led by Dr. Melton-Meaux.
To make a CLHSS services request: Use this form.
Acronym List
- AHC-IE - Academic Health Center Information Exchange - houses secure data for research
- BDAC - Biostatistical Design and Analysis Center - part of CTSI
- BPIC - Best Practice Integrated Informatics Core - part of CTSI
- CDR - Clinical Data Repository - within AHC-IE, managed by BPIC
- C-QODE - Center for Clinical Quality and Outcomes evaluation and discovery - a DOS and Dean’s office resource
- CTSI - Clinical Translational Science Institute
- CLHSS - Center for Learning Health Systems Sciences - Center for rapid QA/QI project integration
- QIP - Quality Improvement Program
Contact and Links
-
Data Navigation and Data Access: Nick Lemke - ntlemke@umn.edu
-
Regulatory Support: Nick or Mary Farnsworth - ewigx005@umn.edu
-
Clinical Trials Support: Mary Farnsworth - ewigx005@umn.edu
-
Grants and Contracts: Hang McLoughlin - mcla0030@umn.ed
-
C-QODE: Alicia Herman - alicia@umn.edu
-
Data Request form: https://cqode.umn.edu/were-ready-whenever-you-are
-
-
BPIC support (data): https://redcap.ahc.umn.edu/surveys/?s=gmfwoj8yGJ
-
BDAC (stats): https://ctsi.ahc.umn.edu/portal/auth/sign_in
-
Note: Must be on campus or using VPN
-
Research Resource Review Request
-
Guide to Submitting Research Review Request - link. - Google Doc
-
Link to request form - Research Resource Review - Smartsheet