Comprehensive Adrenoleukodystrophy (ALD) Clinic
The University of Minnesota is one of the most experienced centers in the world in the assessment and treatment of patients with ALD. We have several clinical trials to treat patients with ALD, and we have published extensively on the clinical aspects of ALD, including biomarkers, oxidative stress, neuropsychology, and imaging.
Our Adrenoleukodystrophy Comprehensive Clinic was established as a multi-disciplinary clinic developed specifically for patients with ALD. We believe the availability of newborn screening will prove extremely important in the identification of patients at risk, and in minimizing the long-term effects of their disease. We are focused on providing assistance to families with a baby diagnosed by newborn screening, including the monitoring important to identify as early as possible adrenal insufficiency and the onset of cerebral disease. However, the clinic is available to adults with ALD and female carriers as well.
Choose M Health Fairview Masonic Children's Hospital
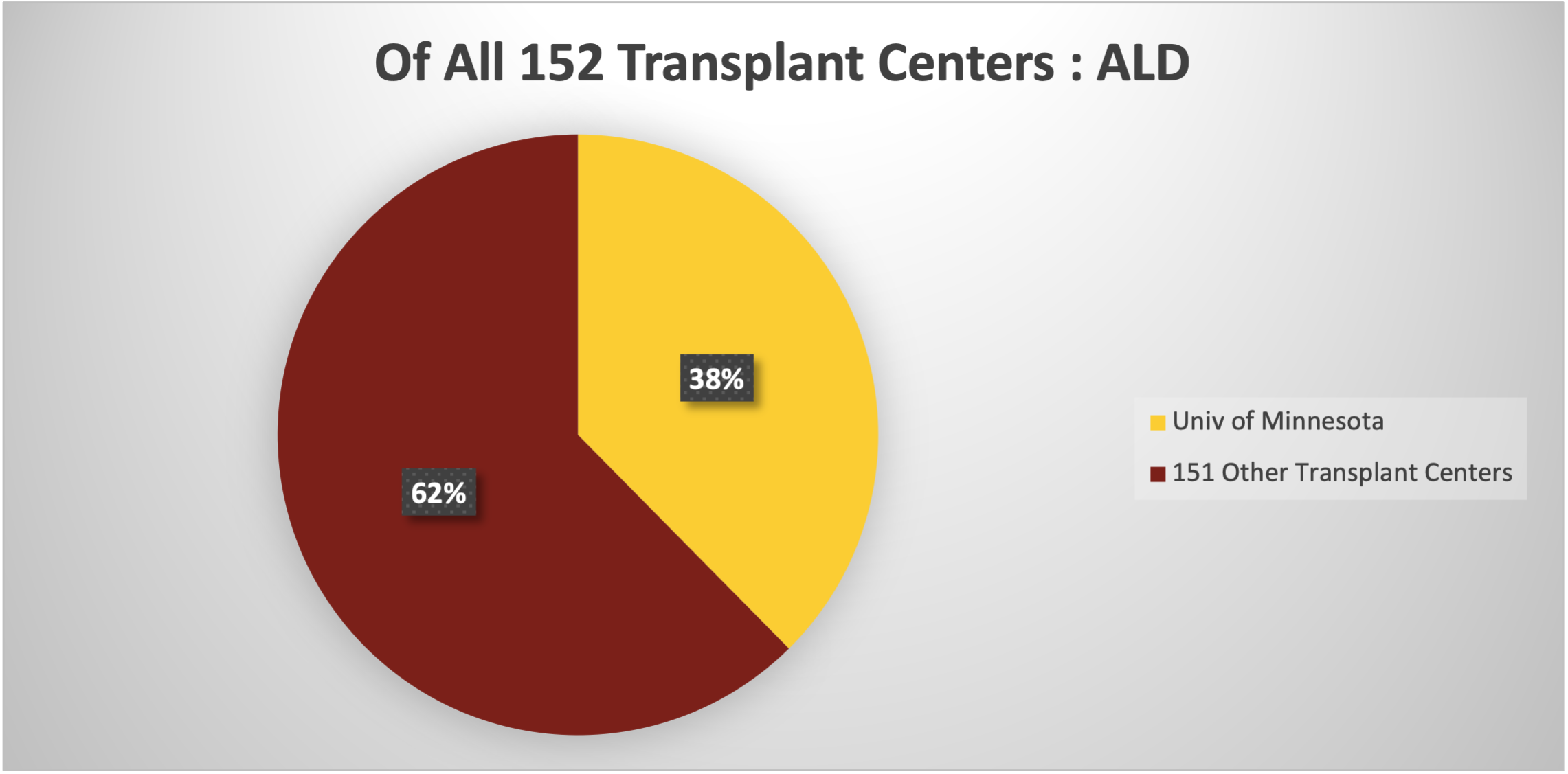
We understand the difficult choices that patients and their families have to make when considering where and how to manage this complex disease, and we are available to assist patients with ALD and their families. The ALD Comprehensive Clinic is located in the Journey Clinic of the MHealth Fairview Masonic Children’s Hospital. It includes experienced providers in endocrinology, neurology, genetic counseling, MRI imaging, neuropsychology and bone marrow transplantation. Our specialists have had the opportunity to care for and follow many patients with ALD, and we are committed to providing the best care available in addressing your child’s complex medical needs. This chart shows how our experience compares to other transplant centers.
Support Our ALD Research

We are grateful to our patients, their families, and the advocacy organizations that make our research possible. Together, we continue to make significant progress in the understanding and treatment of leukodystrophies.
Please contact the Leukodystrophy Center with questions.
leukodystrophy@lists.umn.edu
What is Adrenoleukodystrophy (ALD)?
ALD is a type of Leukodystrophy caused by mutations in the ABCD1 gene located on the X chromosome. The genetic defect affects the ability to break down very long chain fatty acids (VLCFA), and the build up of the VLCFA can be determined at birth.
Affected boys have no issues at birth, but can develop loss of function of the adrenal glands (adrenal insufficiency), which can be determined on blood tests. About 35% of boys also develop changes in the white matter of the brain (leukodystrophy), most commonly between 4 and 10 years of age. The form of the disease (cerebral ALD) may start slowly, but become rapidly progressive, and if untreated, affected boys can die within a few years of the onset of cerebral disease.
The standard treatment is blood stem cell transplant, but outcomes are dependent on finding the disease early. For this reason, newborn screening is being done. Currently many states have added ALD to diseases being screened for at birth (newborn screening). Gene therapy is also being explored for the cerebral form of ALD.
Adrenoleukodystrophy National Registry Study
The ALD National Registry at the University of Minnesota strives to be the world’s largest storehouse of clinical information, MRI scans, and biological samples related to ALD. We believe this will help researchers throughout the world better understand the natural history of the disease, including factors related to the onset and progression of disease. If you are interested in enrolling, the Registry can be accessed here.
Clinical Research and Therapy
Gene Therapy
Qualified Treatment Center for Cerebral ALD
This is a multi-center, long-term safety and efficacy follow-up study for subjects with cerebral adrenoleukodystrophy (CALD) who have received Lenti-D Drug Product in a parent clinical study.
Transplant Therapy
This is a Phase II study for the use of T-cell replete reduced intensity conditioning (RIC) haploidentical donor allogeneic hematopoietic cell transplantation (HaploHCT) for individuals with high-risk non-malignant diseases who lack a suitable HLA-matched sibling donor.
MT2013-31: Allo HCT for Metabolic Disorders and Severe Osteopetrosis
This single-institution, phase II study is designed to test the ability to achieve donor hematopoietic engraftment while maintaining low rates of transplant-related mortality (TRM) using busulfan- and fludarabine-based conditioning regimens with busulfan therapeutic drug monitoring (TDM) for patients with various inherited metabolic disorders (IMD) and severe osteopetrosis (OP).
Other
Adrenoleukodystrophy National Registry Study
This is a prospective, non-therapeutic protocol designed to create and maintain a registry of participants with Adrenoleukodystrophy (ALD) and known/presumed carriers of ALD. This study also involves maintaining a prospective biorepository to collect and store buccal swab, blood, stool and urine samples as well. In this protocol, pediatric (including infants), adolescents and adult patients with confirmed or presumed ALD (based on positive VLCFA testing and/or confirmed mutation) will be offered potential study participation.


