Mucopolysaccharidosis (MPS) Center
WORLD RENOWNED EXPERTISE IN RESEARCH AND CLINICAL CARE FOR HURLER SYNDROME
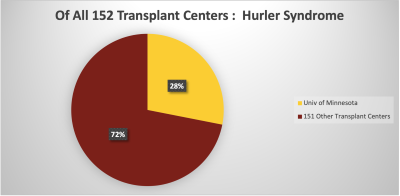
Our MPS Center is internationally recognized for our expertise, innovative treatment approaches, and Hurler Syndrome research. We are here to serve families touched by these disorders as best we can. The University of Minnesota is the first institution in the United States to perform transplants for these disorders, and we have performed well over 250 transplants for Hurler syndrome thus far. We maintain an ongoing commitment to moving the field forward. Patients are cared for by experts in the areas of:
- Audiology
- Blood and marrow transplantation
- Cardiology
- Ear, nose and throat
- Endocrinology
- Genetics
- Neurology
- Neuropsychology
- Neuroradiology
- Neurosurgery
- Ophthalmology
- Orthopedic surgery
- Pulmonology
This multidisciplinary team approach ensures that the care we provide best meets the unique needs of each patient and family. We assist with establishing a diagnosis, providing the best therapy available, and providing multidisciplinary assessments to anticipate difficulties and optimize care.
Contact Us
Clinic Appointments:
(612) 273-2800, option 2
For more information or questions about the Mucopolysaccharidosis (MPS) Center please contact mps@lists.umn.edu
For Professionals
Professionals can earn CME/CNE credit by viewing CheckRare's four courses (Diagnosing MPS I, Treating MPS I, Newborn Screening, and Genetic Counseling) lead by Paul Orchard, MD.

The 24th Dr. William Krivit Lectureship in Pediatrics
Coming May, 2025. Check back for more information!
Research
MT2018-18: Sleeping Beauty Transposon-Engineered Plasmablasts for Hurler Syndrome Post Allo HSCT
This is a single center, Phase 1/2 study in which patients with Hurler syndrome who have previously undergone allogeneic hematopoietic stem cell transplantation are treated with autologous plasmablasts engineered to express α-L-iduronidase (IDUA) using the Sleeping Beauty transposon system.
MT2013-31: Allo HCT for Metabolic Disorders and Severe Osteopetrosis
This single-institution, phase II study is designed to test the ability to achieve donor hematopoietic engraftment while maintaining low rates of transplant-related mortality (TRM) using busulfan- and fludarabine-based conditioning regimens with busulfan therapeutic drug monitoring (TDM) for patients with various inherited metabolic disorders (IMD) and severe osteopetrosis (OP).
Read More
Newborn Screening
In 2016, mucopolysaccharidosis type I (MPS I) was added to the Recommended Uniform Screening Panel (RUSP). The Secretary of the Department of Health recommends newborn screenings in the United States include their RUSP list of disorders. The list of states screening for MPS I is expanding on an ongoing basis. Each state decides which diseases to screen, therefore testing is not universal. The ability to identify patients with MPS I soon after birth allows for early intervention, which we believe will open doors to more effective therapy. This early intervention is an active area of focus for our group and our clinical research.
 Our multidisciplinary team of healthcare professionals at the University of Minnesota Masonic Children’s Hospital collaborates with the Minnesota Department of Health Newborn Screening Program (MDH-NBS). Together we address the varied and long-term spectrum of clinical needs for patients with Hurler syndrome.
Our multidisciplinary team of healthcare professionals at the University of Minnesota Masonic Children’s Hospital collaborates with the Minnesota Department of Health Newborn Screening Program (MDH-NBS). Together we address the varied and long-term spectrum of clinical needs for patients with Hurler syndrome.
 Has your baby received a positive newborn screening test or a biochemical diagnosis of MPS I/Hurler syndrome? What this means to your family and how you should proceed can be complex and confusing. We're here to help, no matter your location. Please email the Mucopolysaccharidosis (MPS) Center (mps@lists.umn.edu).
Has your baby received a positive newborn screening test or a biochemical diagnosis of MPS I/Hurler syndrome? What this means to your family and how you should proceed can be complex and confusing. We're here to help, no matter your location. Please email the Mucopolysaccharidosis (MPS) Center (mps@lists.umn.edu).