Family from Minneapolis Provides Evidence Linking an Inherited Mutation with Cancer and Leukemia Risk
Aging is linked to mutations in normally dividing cells, including the stem cells that give rise to blood cells. Certain changes of blood cells may predict the development of cancer and other age-related diseases. During normal cell division, DNA-replication generates a copy of each chromosome. However, this process normally does not copy where the telomere is located, a region of repetitive DNA sequences at the end of a chromosome, sometimes called the “chromosome cap”. Telomeres safeguard the ends of chromosomes from becoming frayed or tangled. Each time a cell divides, the telomeres become somewhat shorter. This process results in the loss of base pairs of telomeric DNA with every cell division.
As telomeres shorten, they eventually reach a critical threshold triggering a DNA-damage reaction inducing replicative senescence, thus inhibiting further cell divisions. In other words, telomeres become so short that the cell can no longer divide successfully, and that cell dies in the process of aging.
However, the action of telomerase, a specialized reverse transcriptase enzyme adds telomere repeat sequences to the end of telomeres. Stem cells of proliferative tissues, such as the skin and blood, can also express telomerase, which allows them to divide and replicate their DNA multiple times. In prior studies it has been shown that long telomeres extend the replicative potential of cultured cells, which raises the possibility that mutations that prevent telomere shortening may influence specific aging phenotypes and disease risk.
While telomere shortening is a recognized cellular aging mechanism, and short telomere syndromes cause age-related disease, whether long telomere length is beneficial (“a fountain of youth”) is yet to be determined. In a paper published in a recent issue of The New England Journal of Medicine entitled Familial Clonal Hematopoiesis in a Long Telomere Syndrome, doctors examined the clinical and molecular features of aging and cancer in persons carrying a mutation in a new telomere gene called POT1. “This paper would not have been possible without the help from a large multi-generational family from Minneapolis,” said Emmanuel Antonarakis, MD, University of Minnesota Medical School, Associate Director of Translational Research, Division of Hematology, Oncology, and Transplantation. “This family helped to provide most compelling evidence linking germline POT1 mutations to long telomeres, cancer predisposition, and a condition called CHIP (clonal hematopoiesis of indeterminate potential). This work represents a paradigm shift, and proposes a novel mechanism of human carcinogenesis. We are very grateful to this Minnesota family for their dedication to this research, in order to shed light on this new syndrome and to help other families throughout the world with this same genetic mutation” said Antonarakis. POT1 mutations were earlier shown to inhibit the progressive shortening of telomeric DNA that normally occurs with each cell division. The discovery that the POT1 gene is associated with multiple cancer types as well as CHIP suggests a critical role for telomere maintenance in human cancers, challenging the notion that delayed aging is always a good thing.”
Signs of Aging in our Blood
A hematopoietic stem cell can develop into different types of blood cells and when that process starts making cells with the same genetic mutation, something called clonal hematopoiesis is happening. Dr. Antonarakis and his colleagues, joining a team from Johns Hopkins University led by Mary Armanios, MD, found that excessively long telomeres were prone to lymphoid and myeloid clonal hematopoiesis in a pattern of inheritance characteristic of some genetic disorders that showed an increased prevalence with aging. However, the details of how the process of telomerase repeat addition is stimulated in this context still remains unclear, and remains a topic for future research. Drs. Armanios and Antonarakis determined that alteration in DNA that occur after conception, somatic driver mutations, arise during the early decades of life and their long-lived heredities tolerate a high mutation burden of an extended replicative history. The loss of the tumor-suppressor mechanism of telomere shortening supports the expansion of low genetic diversity, clonal populations, which may explain the elevated risk of cancer and CHIP.

Drs. Armanios and Antonarakis found that their data supports the hypothesis that long telomere length convenes a benefit for the endurance of these abnormal telomere clones into adulthood. Multiple genes regulate telomere length, and therefore variants in genes account for the familial clustering of these disorders and their associations with the risk of blood-based neoplasms as well as solid-tumor malignancies. An increased incidence of clonal hematopoiesis has also been documented among persons with the inheritance patterns of single gene diseases, Mendelian short telomere syndromes. However, the spectrum of mutations is distinct, with a secondary mutation that restores the original replication, somatic-reversion mutations, that offset the inherited defect being most common. These observations, together with research findings, advocate that the germline genetic background encourages the spectrum of mutations under clonal selection in hematopoietic compartments.
Understanding Aging and Cancer Biology
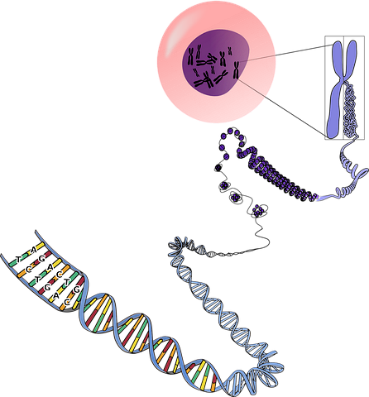
The study offers insight into the inheritance pattern of single gene disease context for understanding the biology that underlies population-based observations linking common variants near the gene encoding telomerase reverse-transcriptase, which are associated with long telomere length, with the risk of somatic mutations in hematopoietic stem cells in an individual without a detectable hematologic cancer and nearly all solid-tumor malignancies. The neoplastic predisposition described offers a paradox at the intersection of aging and cancer biology. Long telomere length, the result of an invulnerability to telomere shortening and increasing telomere length with age, manifests as delayed replicative senescence. Concurrently, it tolerates clonality, which is characteristically associated with older age. The paradox is apparent in the observations that both short and long telomere lengths look as if to mediate two distinct age-associated disease phenotypes.
Drs. Armanios and Antonarakis venture that the relatively high burden of somatic variants in hematopoietic lineages may be comparable to that of other tissues. POT1 mutations associated with long telomere length showed a predisposition to a familial clonal hematopoiesis syndrome that was connected to a collection of benign and malignant solid neoplasms. The risks of these observable traits was facilitated by drawn-out cellular longevity and by the ability to maintain telomeres over time. Overall, the study uncovered an inherited cancer predisposition mechanism that is distinct from that of mutations affecting tumor-suppressor proteins and oncoproteins conferred by an extended cellular lifespan that supports clonal evolution with aging. And, it serves as an important reminder that there may be unintended consequences of drinking too frequently from the fountain of youth.
Dean Patterson, Editor
Division of Hematology, Oncology, and Transplantation
deanofwriting@hotmail.com
References
DeBoy EA, Tassia MG, Schratz KE, Yan SM, Cosner ZL, McNally EJ, Gable DL, Xiang Z, Lombard DB, Antonarakis ES, Gocke CD, McCoy RC, Armanios M. Familial Clonal Hematopoiesis in a Long Telomere Syndrome. N Engl J Med. 2023 May 4. doi: 10.1056/NEJMoa2300503.
Vassiliou George. (2023) Telomere Length and Clonal Hematopoiesis. N Engl J Med DOI: 10.1056/NEJMe2303022.