HIV Research Seeks Answers for Patients Affected by Impaired Cognitive Function
COVID-19 may be today’s medical crisis, but another virus at the center of research worldwide continues to infect about 1.7 million people annually—HIV. Though the virus can be treated with antiretroviral drugs to prevent AIDS, many still suffer from side effects of unknown origin, including impaired cognitive function.
“What most people don’t realize is that almost half of people with HIV have neurocognitive deficits, and there’s no treatment for that impairment,” said Stanley Thayer, PhD, a professor in the Department of Pharmacology at the University of Minnesota Medical School. His lab currently studies HIV-associated neurocognitive disorders (HAND).
“In spite of the fact that combined antiretroviral therapy is really good at suppressing viral replication—in the U.S. most people living with HIV don’t advance to AIDS—living with HIV as a chronic disease is often accompanied by neurological symptoms because the prevalence of HAND remains high,” Dr. Thayer said.
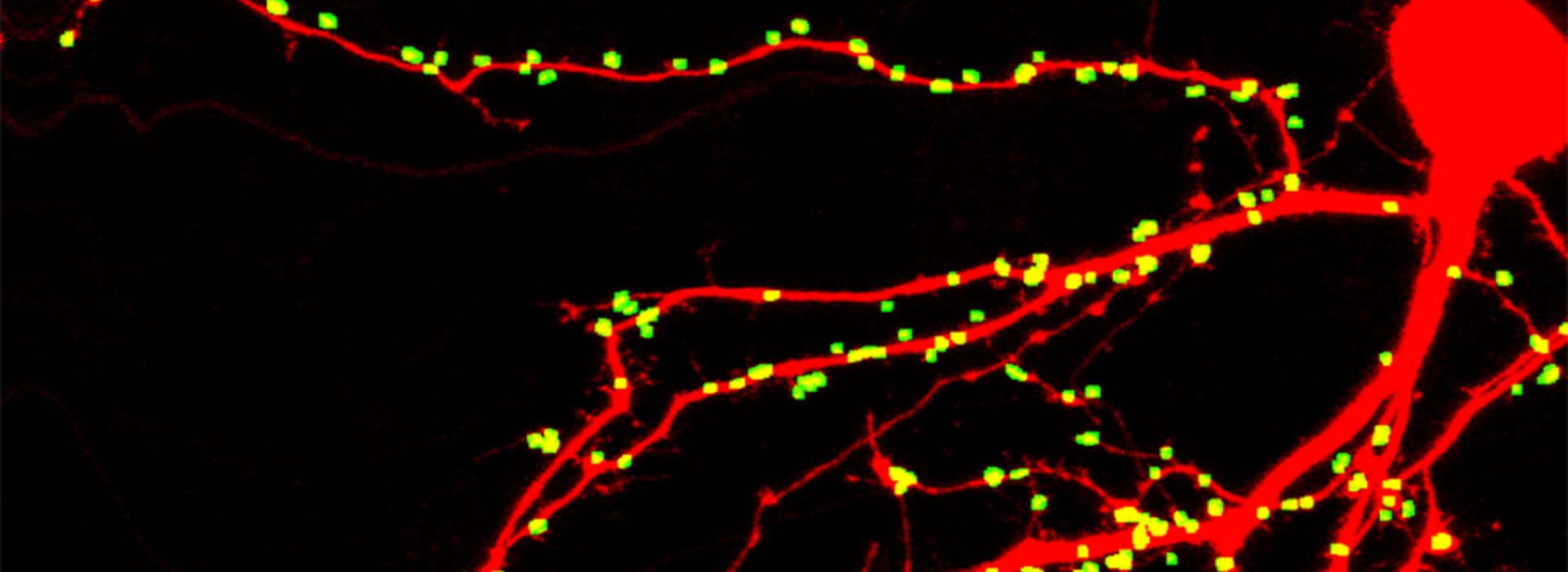
During his 30-year investigation into the basic mechanisms of neurodegenerative disorders, Dr. Thayer has focused on synaptic transmission, learning how one nerve cell in the brain communicates with another. In neurodegenerative conditions, these synaptic connections change.
“Cognitive decline in HAND, like many other neurodegenerative diseases like Alzheimer’s, correlates with the loss of synaptic connections,” Dr. Thayer said. “I’m very interested in tracking these synaptic changes in early stages of diseases, particularly in neuroinflammatory disorders like HAND. One thing to also remember is that neurons routinely, as part of their physiology, change the strength and number of these connections, so in contrast to death, which is irreversible, these connections can come and go. As a pharmacologist, I’m looking at the points where we can intervene and hopefully rescue synapses that have lost connection.”
In 2018, Dr. Thayer and his team made a big discovery—a new target in the brain that drugs could be developed for to rescue synaptic connections and restore cognitive function in those affected by HAND. Using that information, his team developed a highly automated assay, working with “cells in dish,” that allows them to count how many synaptic connections a cell has made, expose it to HIV proteins and see how those connections change over time. In additional preclinical studies, they administered a drug that acts at this target in a mouse that lost synaptic connections after exposure to HIV proteins. The drug actually restored synaptic connections and rescued cognitive function.
“It’s not a good drug to actually think about giving to people right now; it’s more of a tool to study a particular target that might be important,” Dr. Thayer said. “The problem with these types of diseases is you don’t know beforehand who’s going to get it. So, you can’t pre-treat it, and being able to reverse it is a powerful approach. The dream would be to develop a drug that would be tolerated in people that could rescue these connections and reverse the impairment after it’s seen.”
Along with that research, Dr. Thayer and his team received funding this year from the National Institutes of Health to use their highly automated assay and study 20 antiretroviral drugs to see if any of them play a role in HAND as well.
“These drugs are used in different combinations; almost always a three-drug combination,” Dr. Thayer said. “Now, we can look at all of those combinations to see if any of them have adverse effects.”
Between these two related studies, Dr. Thayer hopes his team’s research will provide answers for HAND. But, he believes that understanding the mechanisms behind losing and rescuing synaptic connections will translate across multiple diseases that involve loss of cognitive function.
“Neuroinflammation contributes at some level to most neurodegenerative diseases. In this case, when these viral proteins disrupt the way nerve cells communicate with each other, that loss of control leads to this cascade of events that presents various targets, and many of these play a part in other diseases as well,” Dr. Thayer said. “These fundamental mechanisms, I’m hopeful and I believe, will transfer across many disorders.”