Multi-million Dollar Prostate Cancer Research Effort Led by Dr. Aaron LeBeau
The American Cancer Society estimates about 191,930 new cases of prostate cancer will emerge in the United States during 2020. For a majority of those men, surgery and radiation treatment can effectively cure them if the cancer is non-metastatic. Hormone therapy is currently the first-line treatment for men with metastatic disease. Though initially effective, all men will eventually fail hormone therapy and additional therapies will then be employed.
More men who acquire resistance to all current therapies are developing a highly aggressive subset of prostate cancer called neuroendocrine prostate cancer (NEPC). While there are currently no effective ways to treat NEPC, Aaron LeBeau, PhD, who is an assistant professor in the Department of Pharmacology, is leading groundbreaking prostate cancer research at the University of Minnesota Medical School to find new therapeutics for NEPC.
With the support of three active R01 grants from the National Cancer Institute (NCI), totaling approximately $4 million, Dr. LeBeau is using the NCI grants to focus on non-invasive nuclear imaging and radioimmunotherapy and will build on his previous work published in Clinical Cancer Research in November 2019.
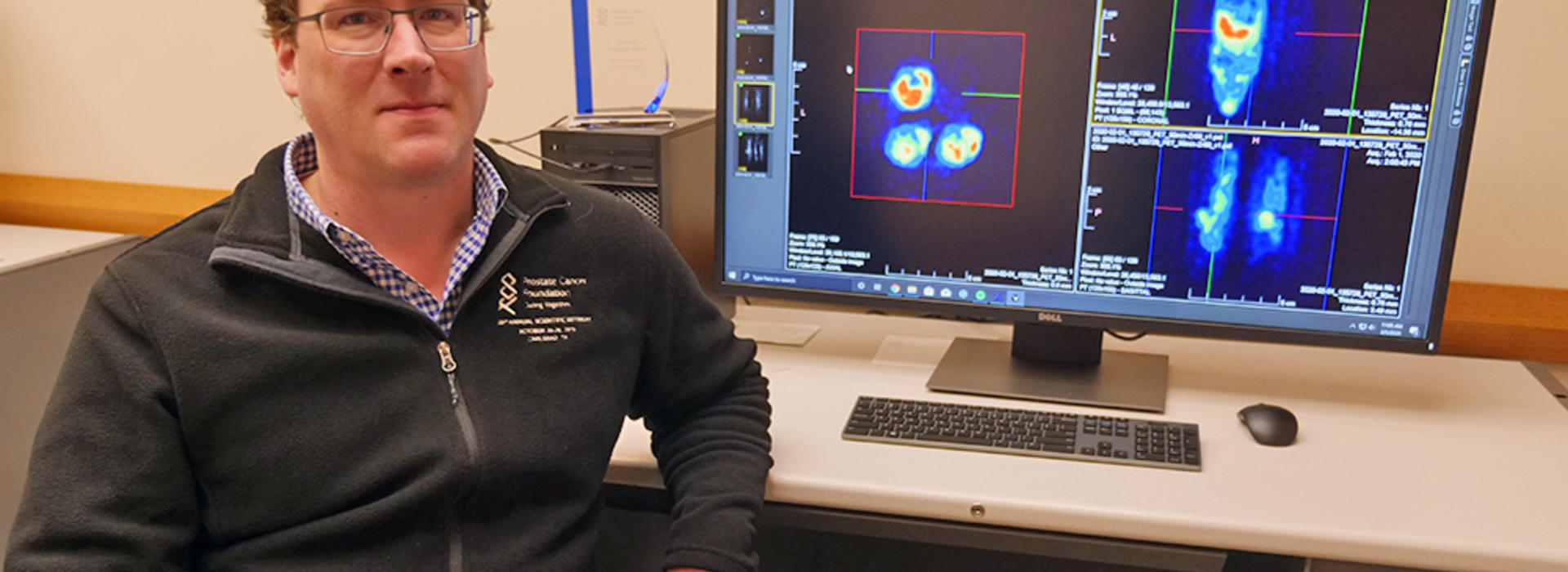
Positron emission tomography (PET) is a non-invasive nuclear imaging technique commonly used in the clinic for assessing disease burden for different types of cancer. PET relies on the use of targeting molecules labeled with radioactive elements that emit a positron when they decay. The positron collides with an electron, resulting in the emission of photons that can be detected by a PET scanner.
“Imaging prostate cancer is historically hard to do because very few targets have been identified for prostate cancer. If we don’t know what to target, we can’t see the cancer cells, and we won’t know where they are or how to treat them,” Dr. LeBeau said.
Dr. LeBeau’s laboratory specializes in antibody phage display, which allows them to rapidly identify antibodies for specific surface markers on prostate cancer cells. Once a novel antibody for the target of interest is discovered, they attach a radioactive element to the antibody and perform PET imaging studies in preclinical animal models of human NEPC. Because of the specificity of the antibody, the signal from the decay of the radioactive element pinpoints the location of the tumor.
“These imaging agents that we develop allow us to actually visualize the cancer cells, so we can know exactly where they are. Once identified, we can annihilate the cancer cells with the same novel antibodies, but now labeled with a therapeutic radioactive element that can shred the DNA of the cell. This technique is called radioimmunotherapy,” Dr. LeBeau said.
This preclinical trial is studying the same antibody structure that the majority of animals in the world have. Dr. LeBeau has also developed a library of unique antibody fragments from members of the camelid family. Llamas and alpacas are members of the camelid family, which have unusual single-domain antibodies called variable-heavy-heavy (VHH) antibodies. VHH antibodies are highly innovative, small and very specific to their target. Other organisms, including sharks, skates and rays, have similar antibody structures to those of the camelids.
“We are identifying new targets all the time for NEPC. We are opening new doors and developing new methods to see and treat this aggressive form of disease, for which there are currently no effective therapies,” Dr. LeBeau said. “Our ultimate goal is to give hope to those who have no hope.”