For Early Treatment of Mild COVID-19, University of Minnesota Trial Shows Hydroxychloroquine Has No Benefit Over Placebo
MINNEAPOLIS, MN- July 16, 2020 – Today, University of Minnesota Medical School researchers published the results from the first randomized clinical trial testing hydroxychloroquine for the early treatment of mild COVID-19 among persons who are not hospitalized.
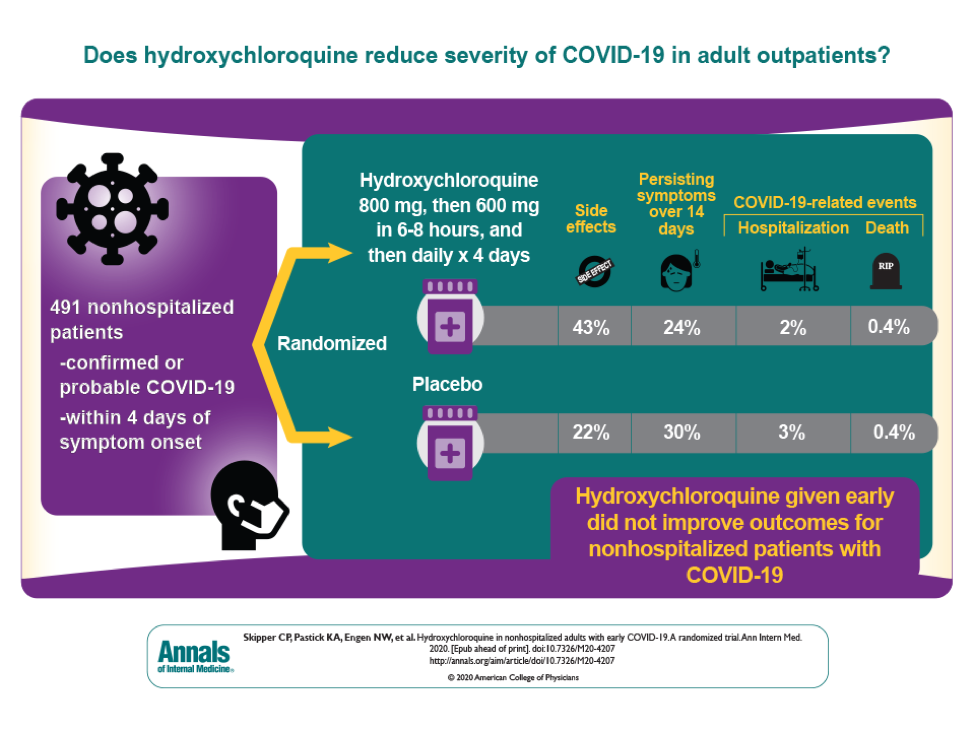
The trial results, published in the Annals of Internal Medicine, determined that hydroxychloroquine did not decrease the severity of COVID-19 symptoms over 14 days any better than a placebo.
The randomized placebo-controlled trial, which rapidly launched on March 22, tested if hydroxychloroquine could decrease severity of COVID-19 symptoms and prevent hospitalization. The trial enrolled 491 non-hospitalized adults from across 40 U.S. states and three Canadian provinces. Participants were enrolled in the first four days of symptoms with 56% enrolled within one day of symptom onset.
Half of the participants received five days of hydroxychloroquine while the other half received five days of a placebo. The trial was a double-blind trial, meaning that neither the participants nor the researchers knew which participants received the placebo or trial drug. Participants were monitored for two weeks to see how quickly symptoms receded and to see who became hospitalized, seriously ill, or passed away.
In addition, there was no benefit in faster resolution of symptom severity among those who also took zinc or vitamin C with either hydroxychloroquine or placebo.
David Boulware, MD, MPH, the senior investigator of the trial and infectious disease physician at the University of Minnesota, launched the trial with the hope that an inexpensive, widely-available, oral medication could treat COVID-19 early in the disease process in order to avert hospitalization.
“This second randomized trial was conducted as a companion to our recently published hydroxychloroquine trial in the New England Journal of Medicine,” said Caleb Skipper, MD, lead author on the paper and infectious diseases fellow studying under Boulware. “Taken together, there is no convincing evidence that hydroxychloroquine can either prevent COVID-19 after exposure or reduce illness severity after developing early symptoms. While disappointing, these results are consistent with an emerging body of literature that hydroxychloroquine doesn’t convey a substantial clinical benefit in people diagnosed with COVID-19 — despite its activity against the virus in a test tube.”
This trial launched within nine days of beginning the project through the work of a team of University of Minnesota infectious disease physicians from the Medical School – Drs. Caleb Skipper, Matthew Pullen, Sarah Lofgren, Mahsa Abassi, Radha Rajasingham, and David Boulware; statisticians Nicole Engen, Ananta Bangdiwala, and Dr. Kathy Hullsiek from the School of Public Health; Pharmacologist Dr. Melanie Nicol from the College of Pharmacy; among many students, trainees and M Health Fairview investigational pharmacy staff. Darlisha Williams and Alanna Nascene oversaw the project logistics. The trial was in collaboration with McGill University, University of Manitoba, and University of Alberta.
###
About the University of Minnesota Medical School
The University of Minnesota Medical School is at the forefront of learning and discovery, transforming medical care and educating the next generation of physicians. Our graduates and faculty produce high-impact biomedical research and advance the practice of medicine. Visit med.umn.edu to learn how the University of Minnesota is innovating all aspects of medicine.
Contact: Kelly Glynn
Media Relations Coordinator, University of Minnesota Medical School
glynn040@umn.edu
414-758-3191