Departmental Research Resource Review Process
Purpose: The Departmental Research Resource Review ensures that resource harmonization and optimization exists for each protocol. This process also provides leadership awareness of the research happening within a unit and offers the department leaders (and administration) an opportunity to provide valuable mentorship and education at pivotal points in research project setup. All protocols regardless of complexity take resources and energy to process and implement.
Effective July 1, 2022, documentation of Departmental Research Resource Review will be required with each initial submission to the IRB for review.
-
Departmental Responsibilities: all Departments in the Medical School will be required to conduct a departmentally-based resource review for all research studies that require IRB approval.
-
PI Responsibilities: Principal Investigators (PIs) will be required to submit their study plans to their respective Medical School Department for a Research Resource Review prior to submitting their study for IRB approval.
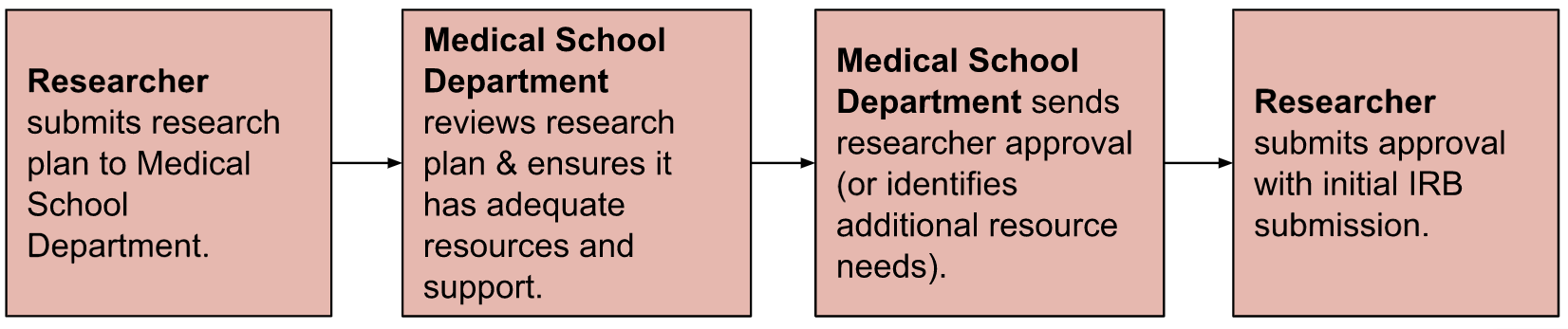
When is a Departmental Research Resource Review Required?
As of July 1, 2022, all researchers in the Medical School will be required to undergo a departmentally-based resource review process for any research studies that require IRB approval. A resource review will be required for the following situations: (1) the PI has a UMN faculty appointment in a Medical School department, (2) the PI is an employee with a P&A appointment that allows them to serve as the PI, or (3) the study requires Medical School resources to advance the project (e.g. faculty, staff, facilities, equipment, or funding).
This review is inclusive of all study types (e.g. retrospective chart review or registry; prospective, observational or treatment; sponsored or non-sponsored; investigator-initiated or business and industry-sponsored) planned for submission for human subjects protection review by either an internal or external IRB.
What do researchers and PIs need to submit and when?
Research teams and PIs will submit their research plan through their Departmental Research Resource Review prior to their initial submission to the IRB for protocol approval.
Typically, this will include submitting a form with study information to their Medical School department. The department will review the study, and provide a signed form or communication indicating approval or denial. If the department notes that more resources are necessary to successfully complete the research plan, the PI, research team, and department will be responsible for making adjustments to ensure that the research project is adequately supported.
When the PI receives an approval letter or form, they will submit this approval along with their IRB submission.
What are the Departmental Process Requirements?
The Medical School requirements for these departmentally-based processes are intended to give each department flexibility, while ensuring each process minimally assesses each protocol for the following:
- 1. Staffing Effort: The PI must allocate adequate effort to lead the study, and the total percent effort across all team members must be enough to complete the study.
- 2. Protocol Funding: The PI must identify the specific funding source/s they will use to complete the study. The funding source/s must be adequate to cover all study costs; TBD is not acceptable. Department leadership (Department Chairs, Vice Chairs for Research, or Division Directors) should sign off on any departmental costs. If a study is over-budget or not fully funded, the Department Chairs (or their designee) signature assures the department will cover those costs.
- 3. Participant Recruitment: The PI must document a plan that demonstrates an ability to recruit and enroll the intended study population.
- 4. Research Priorities: The Department Chair (or their designee) confirms the protocol fits into the research goals of the Department.
The departments have designated at a minimum, two leadership approvers (e.g. Division Directors, Department Chairs or Vice Chairs for Research) to sign off on the resource review for the protocol prior to the PI submitting the protocol to the IRB. Their signature assures adequate resources are available for the study.
What are the Departmental Responsibilities?
All departments within the Medical School have an approved process or partnership plan in place for a research resource review. Faculty researchers are encouraged to reach out to their Department Administrator or Research Manager for more information on department specific review requirements.
As a reminder for researchers, on August 15, 2022, the IRB started checking new IRB submissions in Ethos for documentation of resource review and approval. If you have general questions about this process or who to contact within your department to submit a study for review, please contact Mahrya Johnson (mjohnso@umn.edu).
How Will We Assess the Effectiveness of This Process?
The effectiveness of this process will be assessed over time. The Research Office is currently working through the metrics data and reporting options. In an effort to provide the highest quality data, please ensure that your studies have been added to OnCore, with a completed minimal footprint and current status. The Research Office anticipates departments will use these data, and with time, adapt their processes during the first year of implementation, while still assessing the four requirements noted above, to ensure their resource review processes are working to improve turnaround time metrics.
What If I Have Questions?
The Medical School Research Office has started a Frequently Asked Questions (FAQ) document for commonly discussed topics. Please contact Jane Welter (colli691@umn.edu) with any questions.